Cidofovir
Cidofovir, also known as Vistide™, is a newer anti-viral drug originally developed for use in a condition called cytomegalovirus (CMV) retinitis. It is active, however, against other DNA viruses, including the human papillomavirus (HPV) that causes recurrent respiratory papillomatosis (RRP), aka laryngeal papillomatosis.
Given systemically (into the entire body via a vein) at the standard recommended dose, it is a “toxic” drug. Fortunately for persons with laryngeal papillomatosis, a tiny, non-toxic, fractional dose (about 1% of the systemic dose) can be injected into the papillomas themselves, thereby sparing the patient the toxicity of a systemic dose level.
Are there prior reports on the use of cidofovir for laryngeal papillomatosis?
Several reports have appeared concerning the use of cidofovir against HPV infection. Here is a brief summary of some early reports:
- In 1995 Van Cutsem et al injected this medication into a papilloma of the esophagus. A total of 7 injections under general anesthesia over a 5 month period caused the lesion to disappear.
- In 1998 Snoeck and co-workers described a group of 17 patients with severe RRP whose lesions were injected every few weeks under general anesthesia. Fourteen appeared to be “cured.” Four relapsed but were successfully re-treated. Of the 3 who did not respond as well as the rest, one seemed to have an initial response, and then the papillomas continued to grow in spite of the cidofovir. The second responded partially, and the third remained stable for more than a year after the last injection. No changes were seen in blood chemistry studies, and no patient experienced an adverse reaction at the site of injection.
- Wellens and co-workers reported in 1997 a series, which also included 17 patients, also injected intralesionally. After semi-weekly injections under general anesthesia (between 3 and 15 spaced over between 1 and 13 months), ten of the 17 patients seemed to be “cured.” The remaining 7 patients needed a longer course of treatment, or they recurred after stopping the injections, and had to undergo additional injections.There were no adverse reactions clearly tied to the cidofovir. One patient with known heart disease reported a single post-injection episode of transient angina. Another described headache after each injection. A third had a rash which was felt to be due to medications used for anesthesia, and not to cidofovir. Two patients needed subsequent surgery for larynx cancer. Each was known to have a form of cancer on the pre-cidofovir biopsies.
- Pransky et al (1999) utilized cidofovir injections in 5 children with RRP so severe it was necessitating surgery on average every two weeks, and at least monthly to maintain an airway! These children had also failed to respond to various other medical trials.Under general anesthesia, cidofovir was injected every 2-3 weeks. Although no “cures” were reported, surgical interval was markedly increased from an average of 2 weeks to 3 or more months.
- Wilson et al (2000) published a preliminary report concerning 3 adult patients whose papillomas were injected every 2-4 weeks for a total of between 7 and 15 treatments. Lesions resolved completely in all three patients, but all three suffered minor recurrences a number of months after the last injection. Lesions again regressed with resumption of cidofovir injections. No patient experienced local or systemic adverse reactions.
You said this medication is only approved for a treatment of CMV retinitis. Wouldn’t that make its use for papillomatosis experimental?
Doctors distinguish between investigational use of a new medicine and off-label use. Once a drug has been released to the market for a specific condition, doctors may legitimately and legally utilize it for another purpose—unless that proposed use was found to be “forbidden” in the work used to support the drug’s release.
Examples of off-label use of medication are numerous. Aspirin, first known for treating pain and fever, was later used widely as a blood thinner. Inhaled steroids, first approved for asthma, were soon used “off-label” in the nose for nasal allergy.
Botulinum toxin was initially approved by the Food and Drug Administration (FDA) for an eye condition called blepharospasm. Yet it is in widespread use various other parts of the body, particularly for neurological disorders. We use it a lot, off-label, for a condition called spasmodic dysphonia.
Sometimes an established “new” use for a medication is never officially approved by the FDA, because the drug company’s expense to do all the trials, statistics, paperwork, and so forth required to win approval cannot be financially justified when the market (and corresponding potential return on investment) for that new indication is small. This will likely be the case for a relatively rare disorder such as RRP.
The reports above all mention that injections were done under general anesthesia about twice a month.
Yes, that is true. At BVI, however, we perform most of our injections instead in the videoendoscopy procedure room of our own facilities, rather than in the operating room. This eliminates the need for general anesthesia and should greatly lessen both the inconvenience and discomforts for patients and the cost to insurance companies.
Can you explain the logistics of the injections?
If an individual is in the operating room to remove bulky disease, the final step of the procedure is to inject cidofovir into the point where the papilloma was attached before it was removed. For BVI (videoendoscopy procedure room) injections, most individuals come to BVI unaccompanied.
Initially, we topically anesthetize (numb) the entire mouth, throat, and larynx (voicebox). Then, a long, thin, curved cannula is passed over the base of the tongue and into the larynx. There is a tiny needle at the tip of the cannula with which to inject the medication.
Alternatively, we watch the larynx with a special scope but inject through the skin of the front of the neck, which has also been “numbed.” (This sounds frightening and difficult to most people, but is surprisingly well-tolerated.)
There should be no significant post-procedure soreness, but there may be a short-term increase in hoarseness, just due to the injection of the medication. In 20 minutes or so the numbing wears off, and the person can go home. There will be no requirement for voice rest.
If a patient has a very strong gag reflex, an oral, or sometimes intravenous, sedative will be administered before and/or during each procedure. In this case, a companion must accompany the individual and drive him or her to and from the appointment.
The current plan is to complete a series of three injections, then assess response to see if further injections ought to be done. Or, when an individual needs surgical removal of papillomas, to inject the first time in the O.R. right after removal, and then two additional times, at two or three week intervals, for a total of 3 injections.
Can you review any potential harm that could come to me? I’m especially worried about the drug’s toxicity.
- If you weighed 150 pounds and had CMV retinitis, the recommended systemic dose of cidofovir would be about 350 mg. At this dose, there is some risk of damage to kidneys and a low white blood count. However, we use on average only 2 or 3 mg, that is, about 1% of the systemic amount, and to inject it intralesionally.
- There could be some inflammatory reaction in the larynx, though this has not been confirmed in the experience reported to date. Some believe that if cidofovir is delivered in concentrated, rather than dilute, form, there is a risk of scarring.
- There are remote risks of reaction to the topical anesthesia.
- In one rat study, there were some mammary tumors (breast) that occurred when cidofovir was given weekly at a dose generally much higher than we will be using. No tumors were seen in rats when the dose was either 4 or 20 times the dose we will be using.
Do you know if insurance will pay for this procedure?
The short answer is that insurance company payment is never a certainty until it actually happens. That said, recently, we have had very little difficulty in getting this medical treatment covered. The best plan is to get the proposed treatment pre-certified, which BVI personnel can help you do.
Keep in mind that if cidofovir is as beneficial to you as it has been to many others, it is in the insurance company’s best interest to pay, so as to avoid the much greater expenses of the operating room.
I’m having trouble with the idea that two patients in one of the studies had cancer.
That does look bad at first. Yet, this worry diminishes greatly when you know that these two patients evidently had cancer in the original biopsy before cidofovir was ever injected. This is not entirely surprising when one remembers that HPV seems to induce cancer on occasion.
In our caseload, we have about 5 patients of an estimated 160 who have developed cancer. To my knowledge, these have all been with HPV type 16. By the way, all have thus far responded beautifully to treatment and are doing well. Thus, it appears at this point that it is the disease, and not this new treatment, which is the risk of cancer.
How do I get started?
First comes the insurance pre-certification process. Then, if your disease is minimal, we sometimes begin cidofovir injections in the videoendoscopy procedure room of BVI to see if your lesions respond. Typically, at least a few injections are done before this determination can be made.
If you currently have a fairly large volume of disease, then the best plan is to go once more to the outpatient operating room, remove the lesions meticulously but conservatively, and inject cidofovir into the base of where the lesions were attached. Thereafter, two additional injections at two to three weeks apart are done to complete a series of three injections. Then we wait to see if the lesions re-grow.
Long-Term Remission or even “cure” of RRP/Laryngeal Papilloma
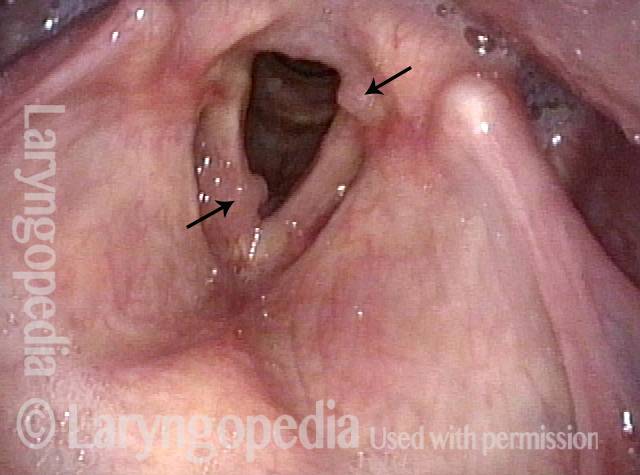
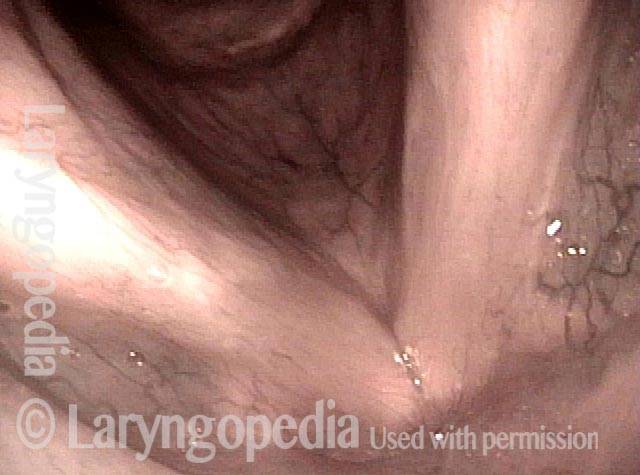
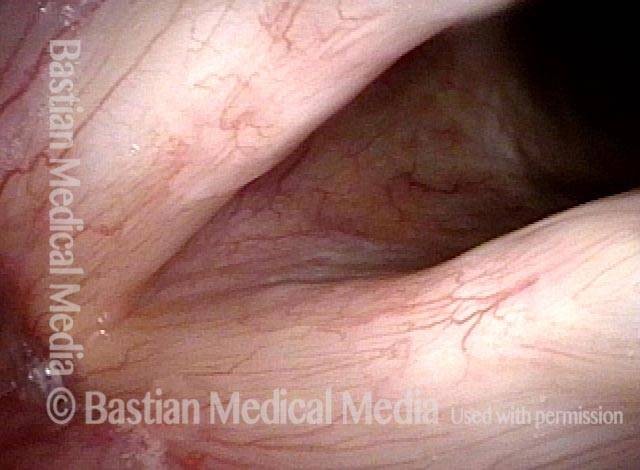
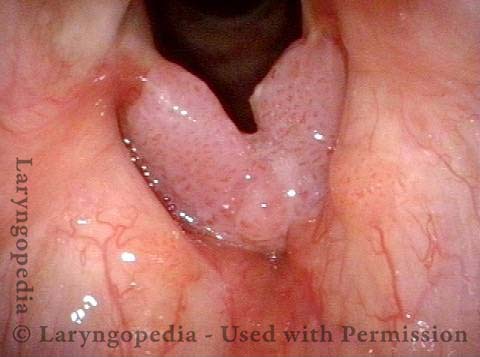
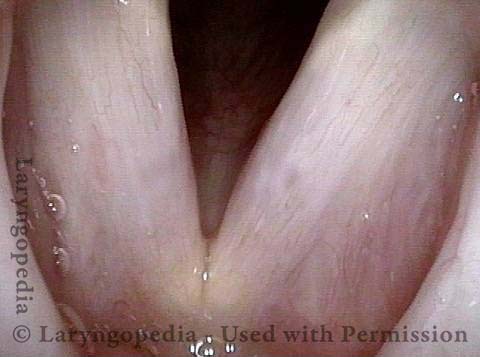
Chronic hoarseness (1 of 6)
Chronic hoarseness (1 of 6)
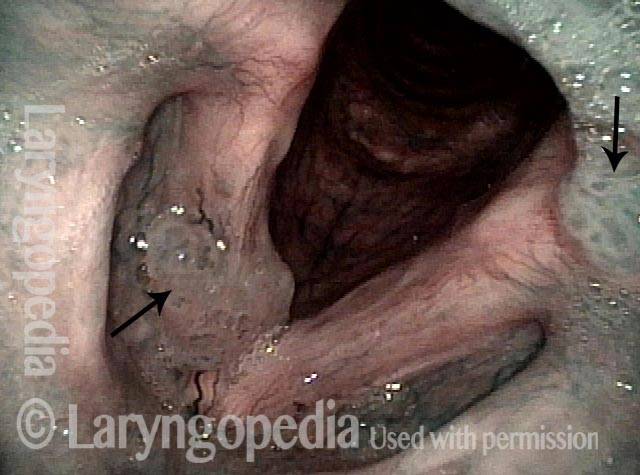
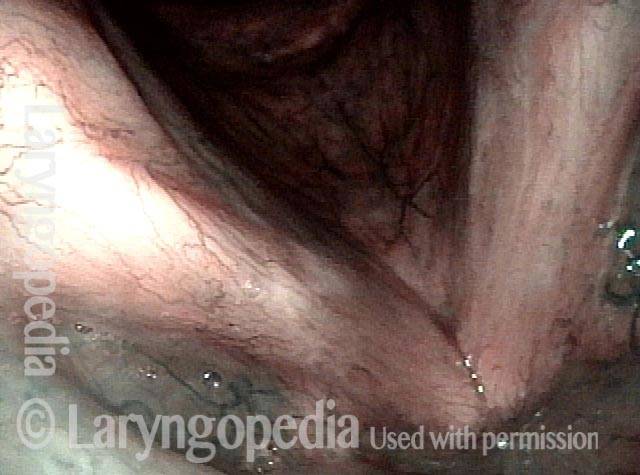
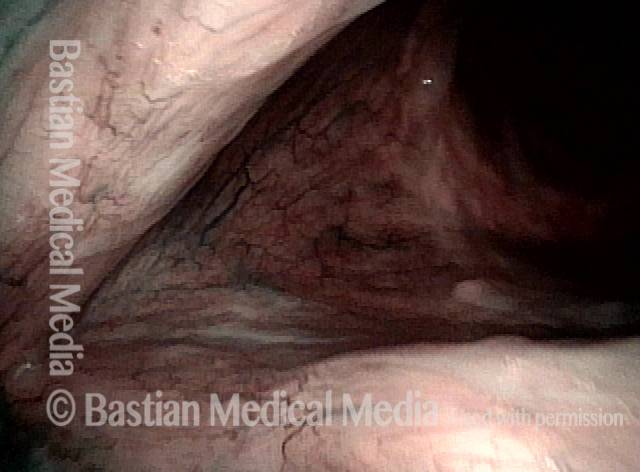
Narrow band light (2 of 6)
Narrow band light (2 of 6)
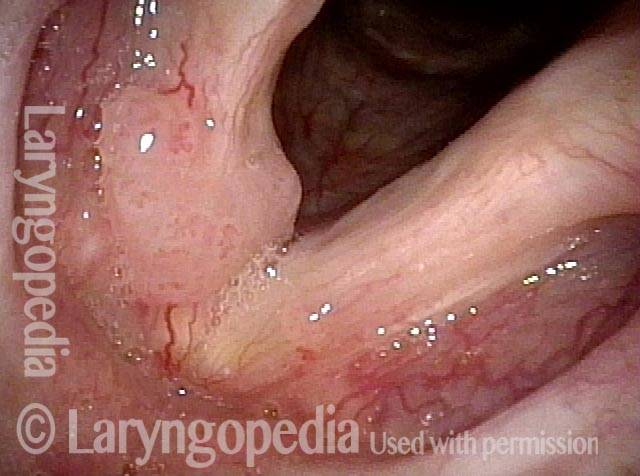
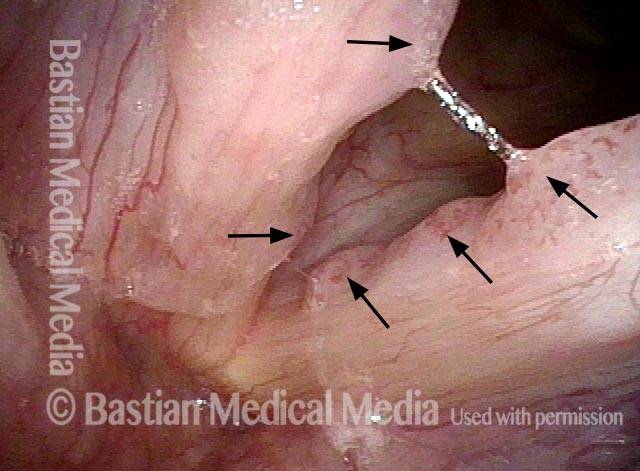
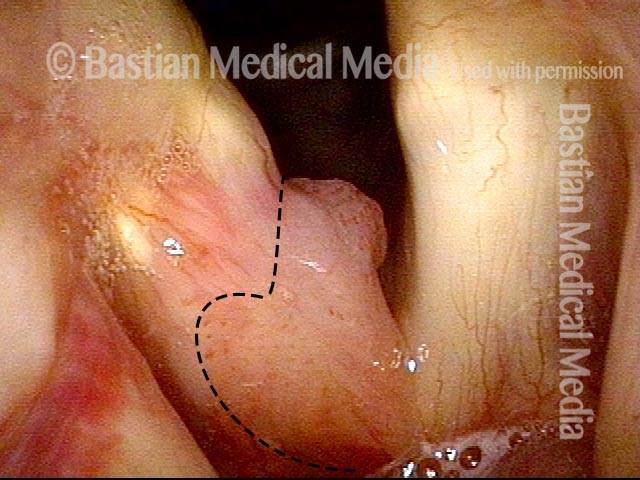
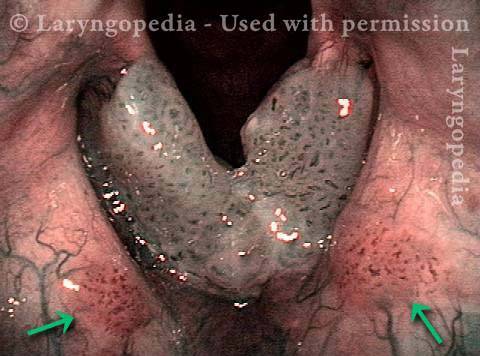
Higher magnification (3 of 6)
Higher magnification (3 of 6)
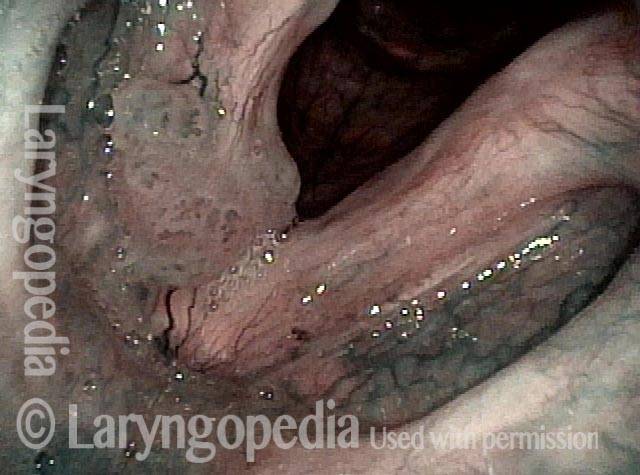
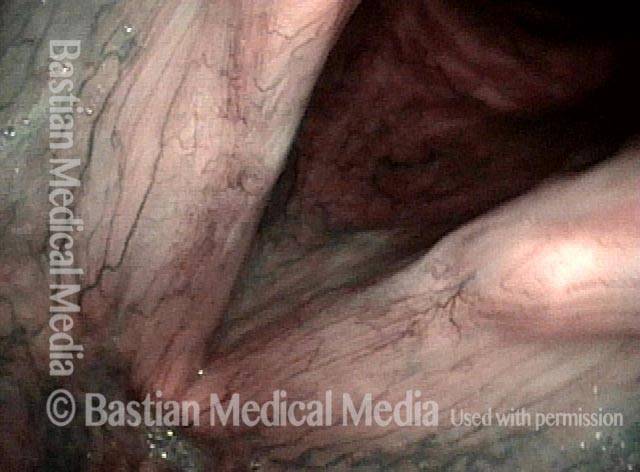
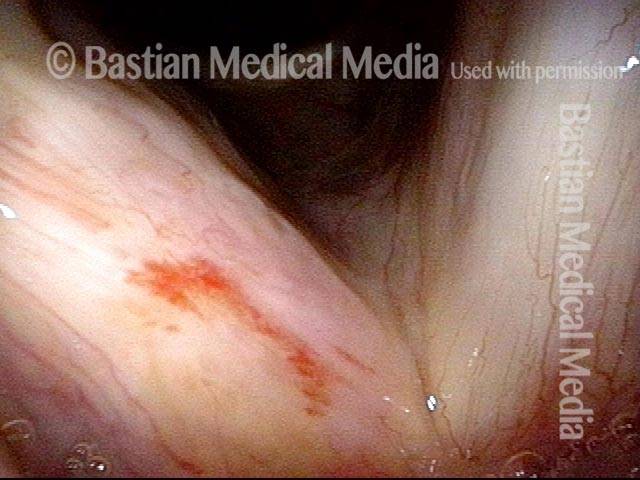
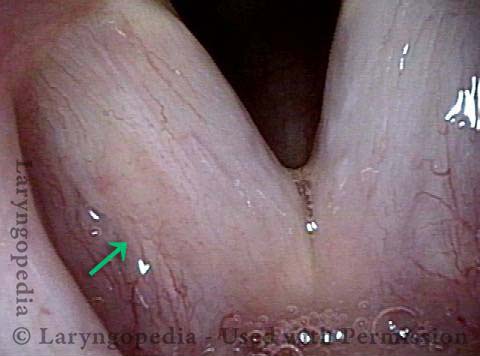
Higher magnification, narrow band lighting (4 of 6)
Higher magnification, narrow band lighting (4 of 6)
Post-operation (5 of 6)
Post-operation (5 of 6)
Post-operation, narrow band lighting (6 of 6)
Post-operation, narrow band lighting (6 of 6)
RRP Cure? Or Just Long Term Remission?
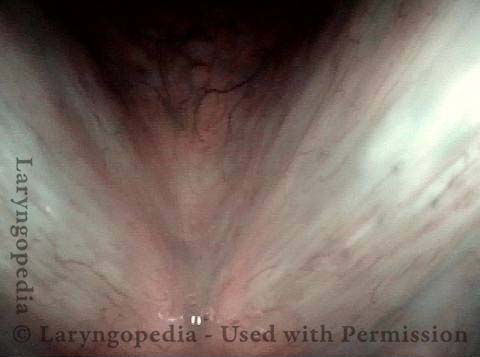
Chronic hoarseness (1 of 4)
Chronic hoarseness (1 of 4)
4 months later (2 of 4)
4 months later (2 of 4)
8 months from start of treatment (3 of 4)
8 months from start of treatment (3 of 4)
Narrow band lighting (4 of 4)
Narrow band lighting (4 of 4)
What “Cured” this Case of RRP? Surgery? Cidofovir? The Patient’s Immune System? All Three?
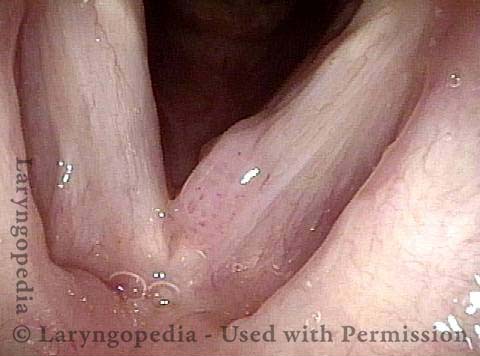
Papilloma (1 of 8)
Papilloma (1 of 8)
Stippling (2 of 8)
Stippling (2 of 8)
One week after surgical removal (3 of 8)
One week after surgical removal (3 of 8)
Cidofovir injection (4 of 8)
Cidofovir injection (4 of 8)
Six months after surgical removal (5 of 8)
Six months after surgical removal (5 of 8)
One week after second removal (6 of 8)
One week after second removal (6 of 8)
4 months later, healed (7 of 8)
4 months later, healed (7 of 8)
3 years later, no sign of papilloma (8 of 8)
3 years later, no sign of papilloma (8 of 8)
Humility Before the HPV Virus—A Recurrence of Papillomas at Ten Years
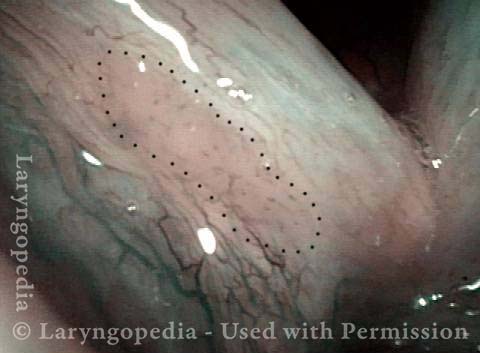
Stippled Vascularity (1 of 8)
Stippled Vascularity (1 of 8)
HPV infection (2 of 8)
HPV infection (2 of 8)
Stippled vascularity (3 of 8)
Stippled vascularity (3 of 8)
HPV vascular effect (4 of 8)
HPV vascular effect (4 of 8)
16 months later (5 of 8)
16 months later (5 of 8)
Is it long-term remission? (6 of 8)
Is it long-term remission? (6 of 8)
Recurrent Papilloma (7 of 8)
Recurrent Papilloma (7 of 8)
Stippled vascularity (8 of 8)
Stippled vascularity (8 of 8)
HPV 18—High Risk Subtype
Papillomas seen (1 of 4)
Papillomas seen (1 of 4)
Stippled vascularity (2 of 4)
Stippled vascularity (2 of 4)
Surgical removal (3 of 4)
Surgical removal (3 of 4)
Cidofovir injection (4 of 4)
Cidofovir injection (4 of 4)
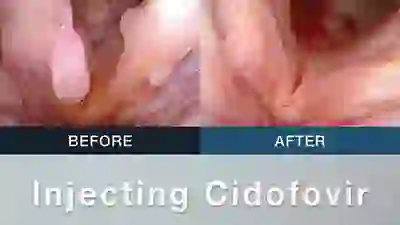
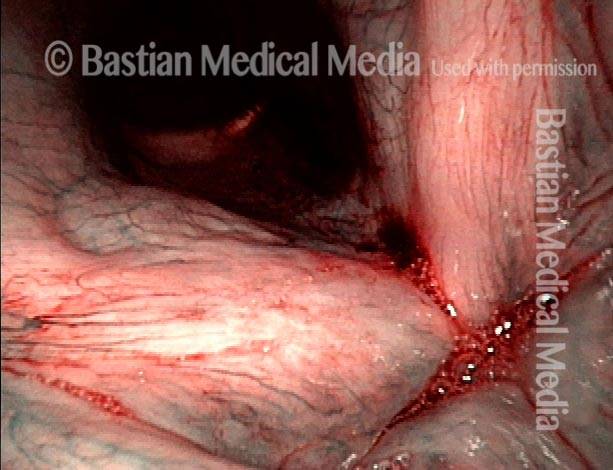
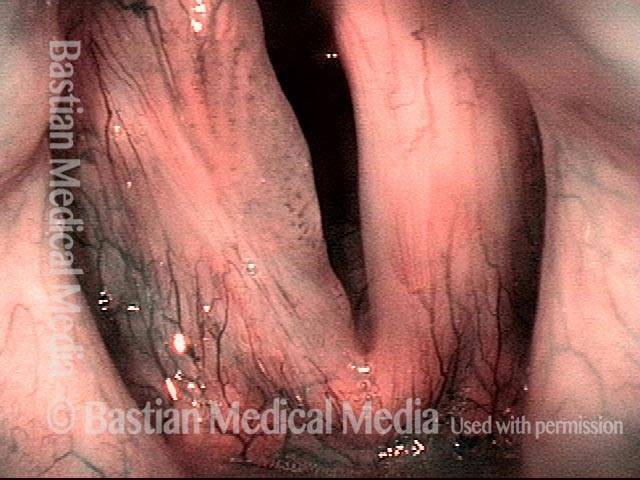
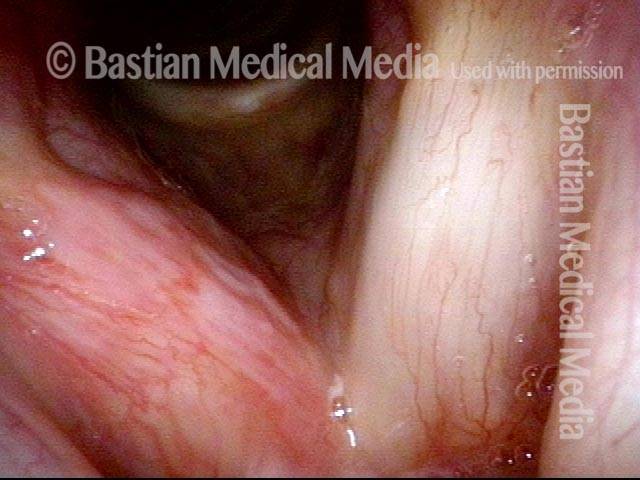
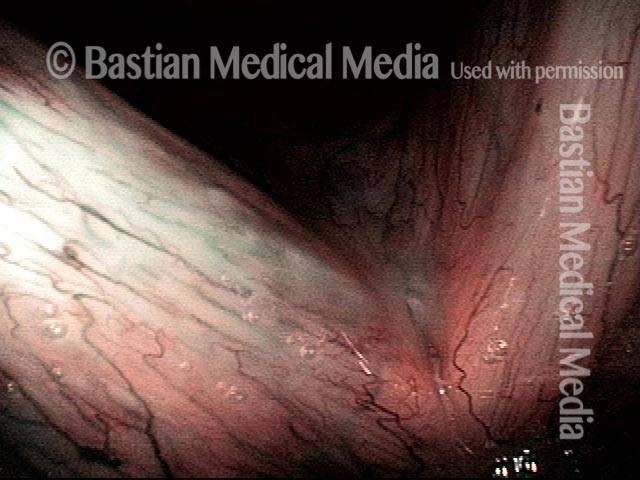
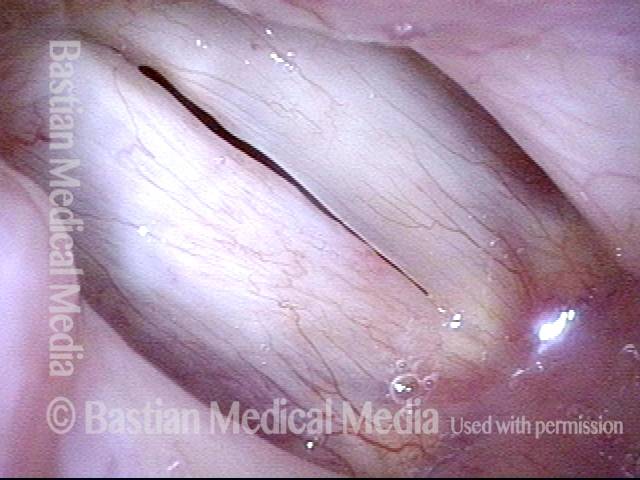
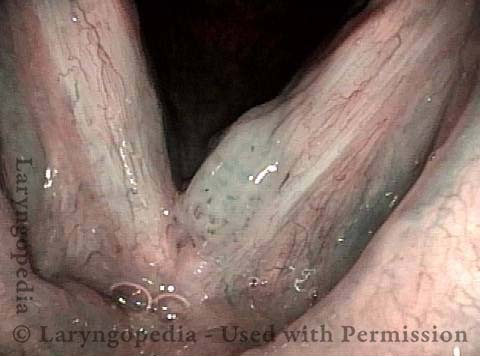
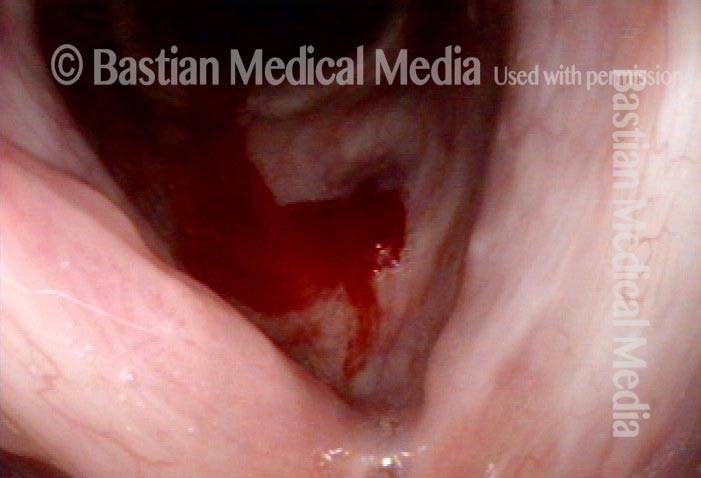
Vocal Cord Injection of Cidofovir
This video portrays a vocal cord injection of Cidofovir following the removal of a papilloma.