Vocal Polyps:
An Actress’ Polyp Before and Hours After Surgical Removal
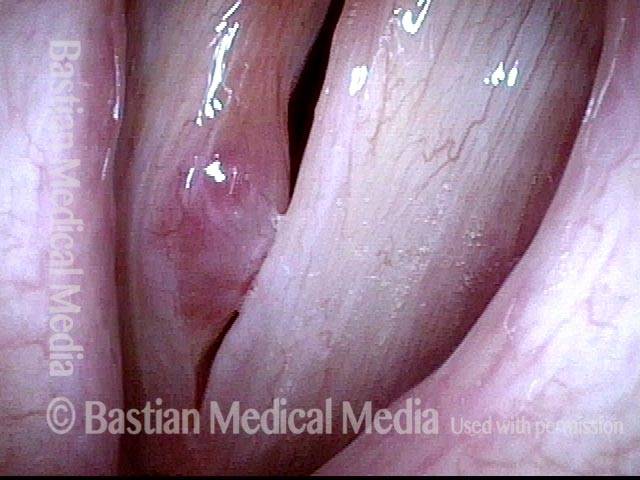
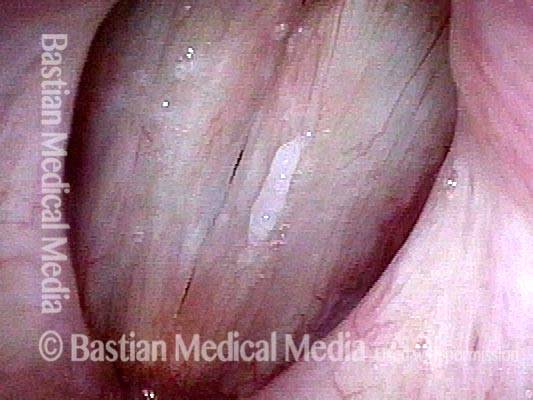
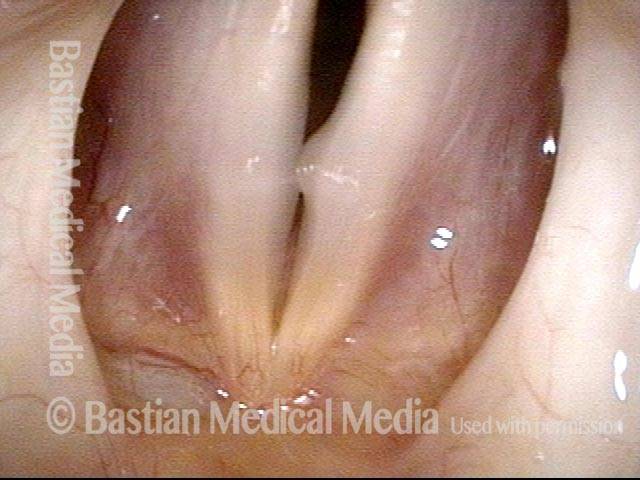
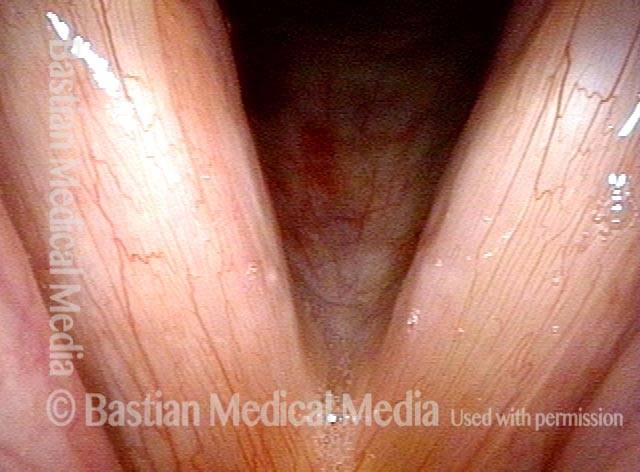
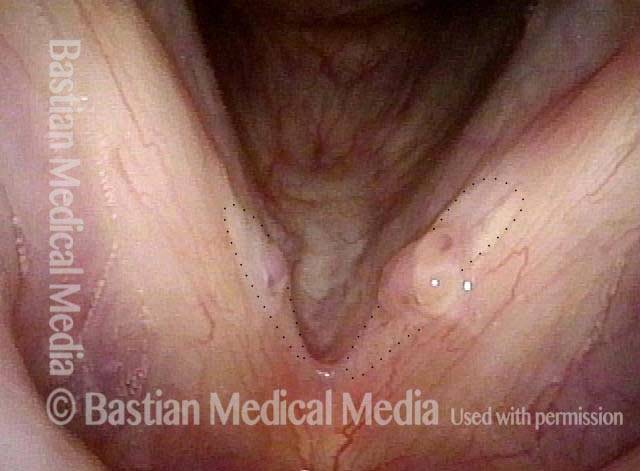
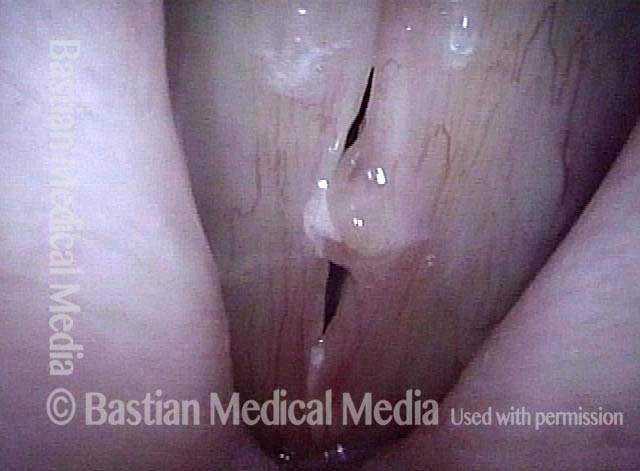
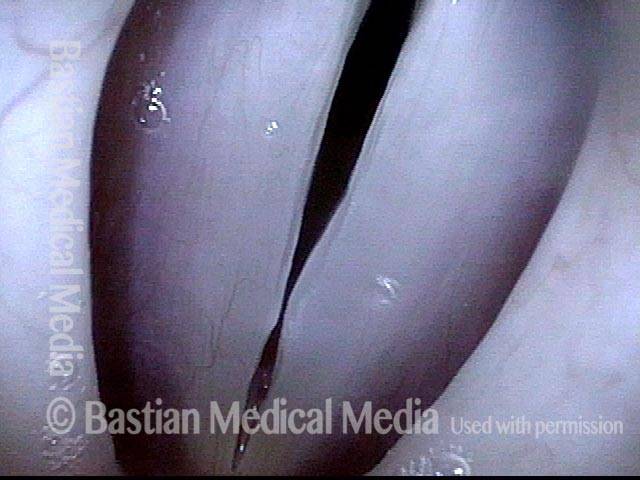
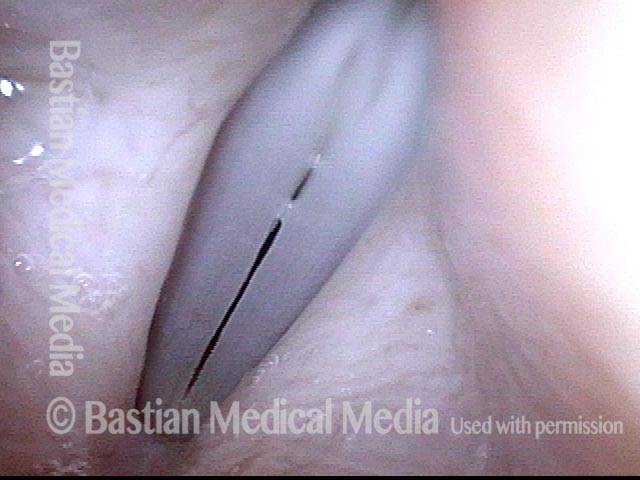
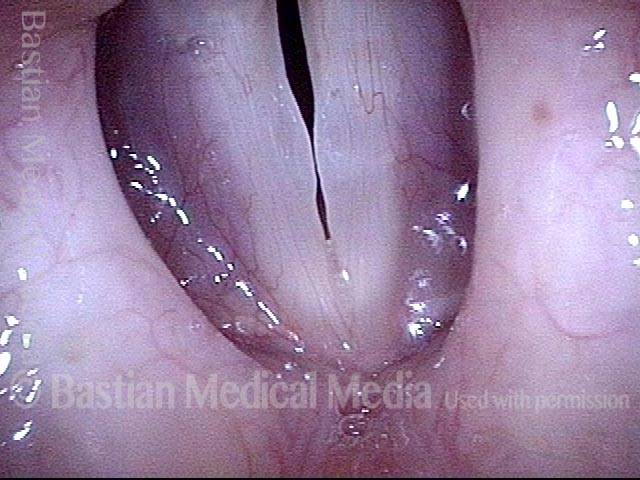
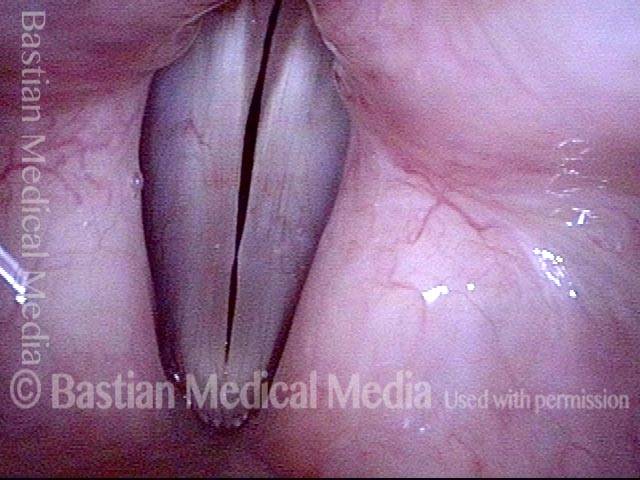
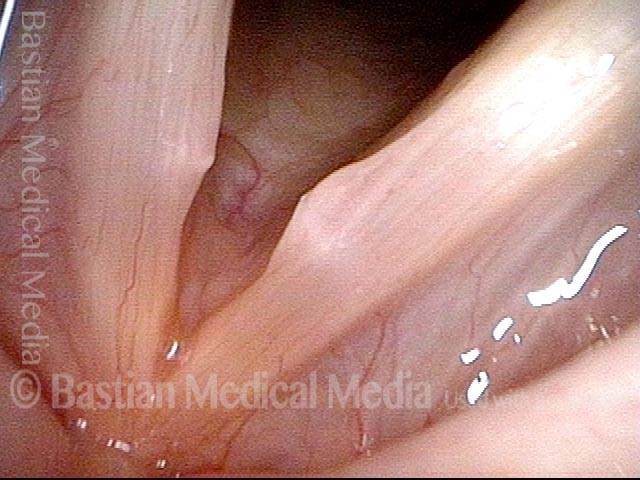
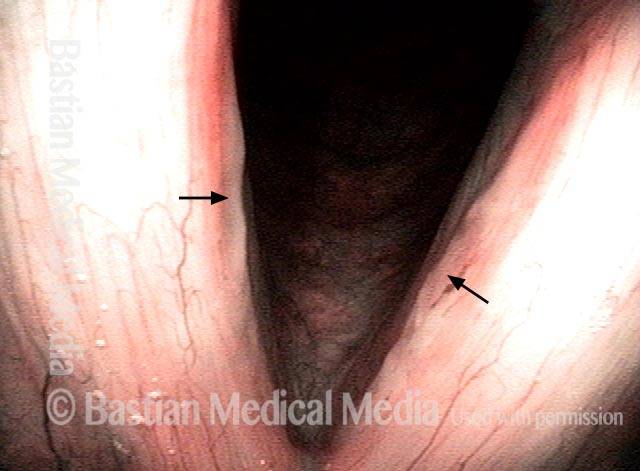
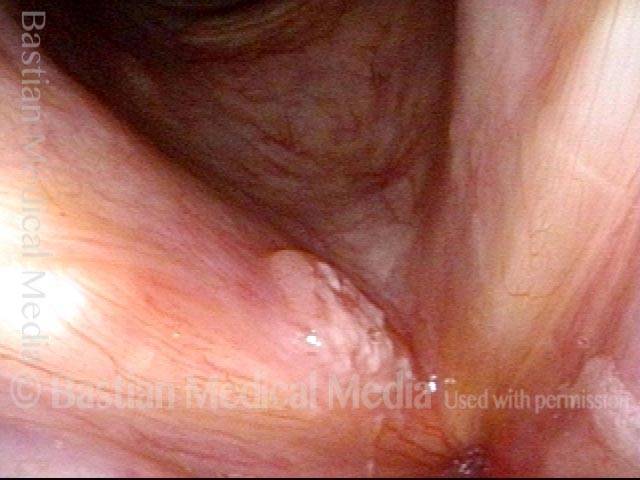
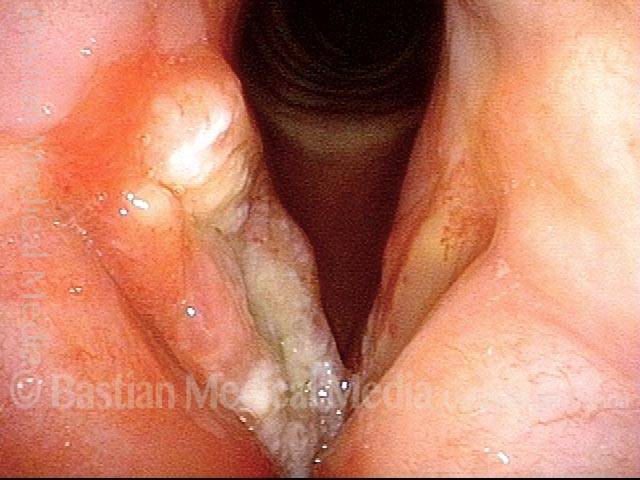
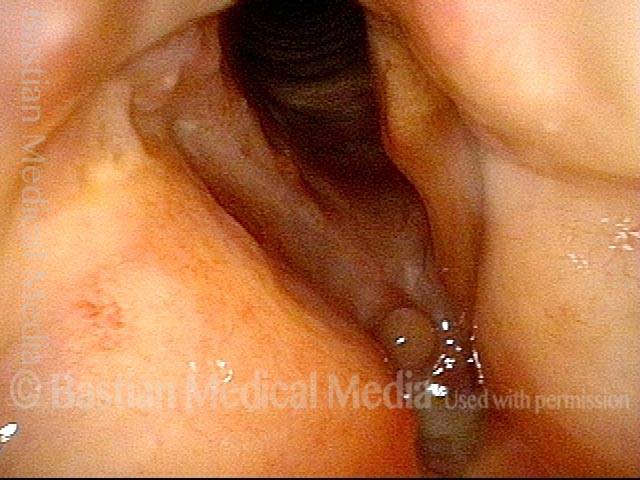
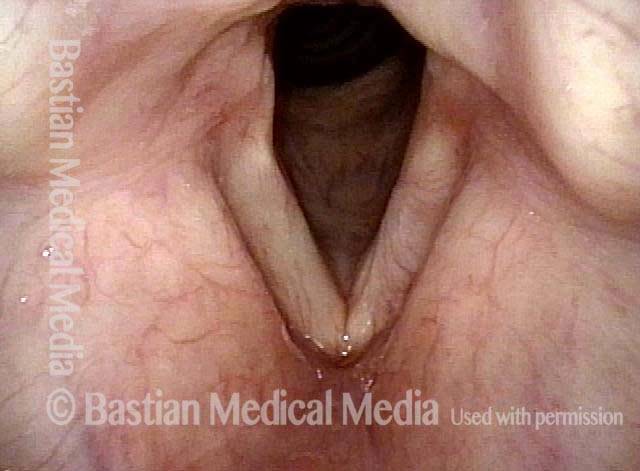
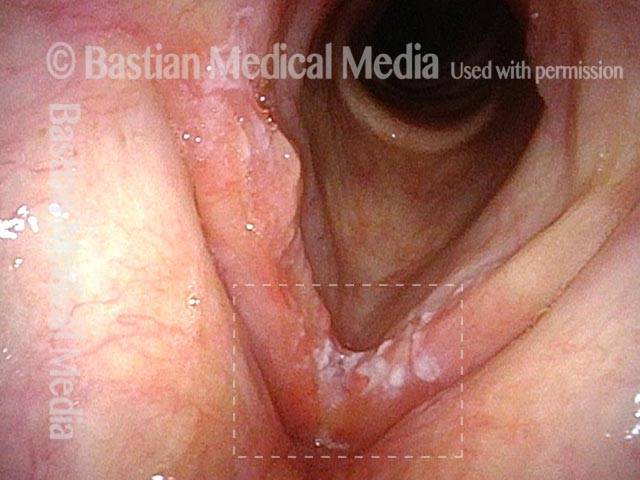
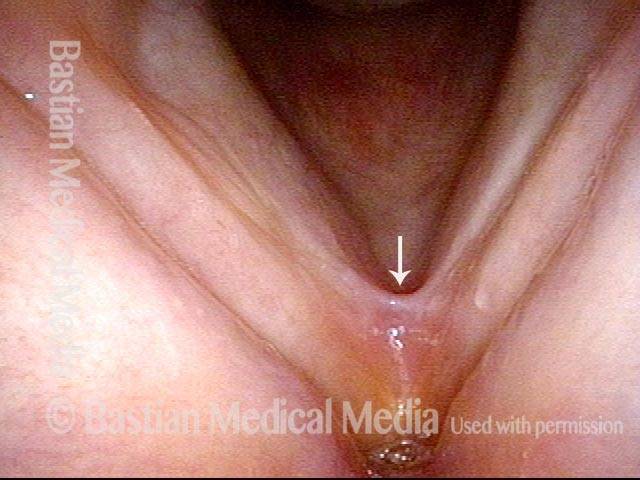
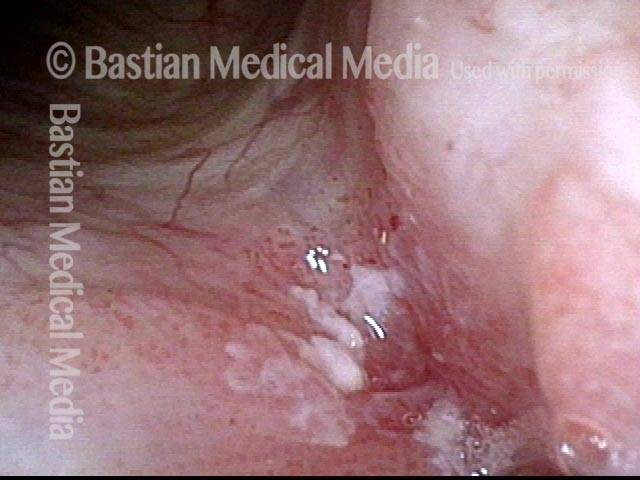
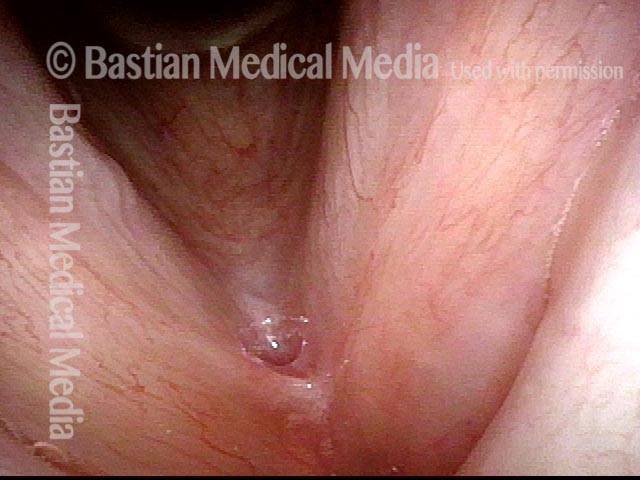
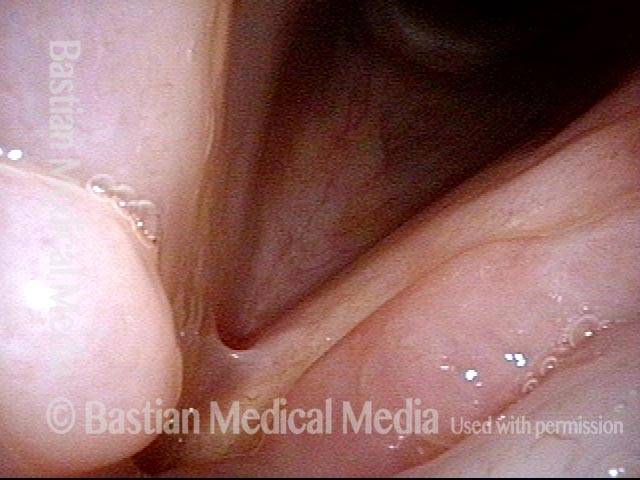
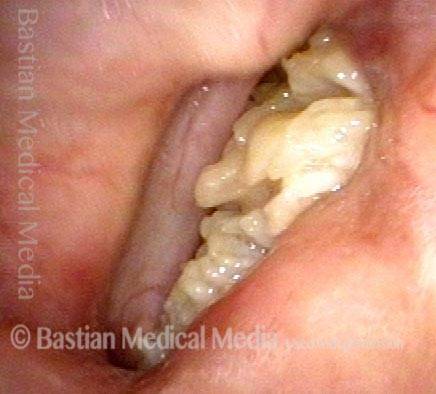
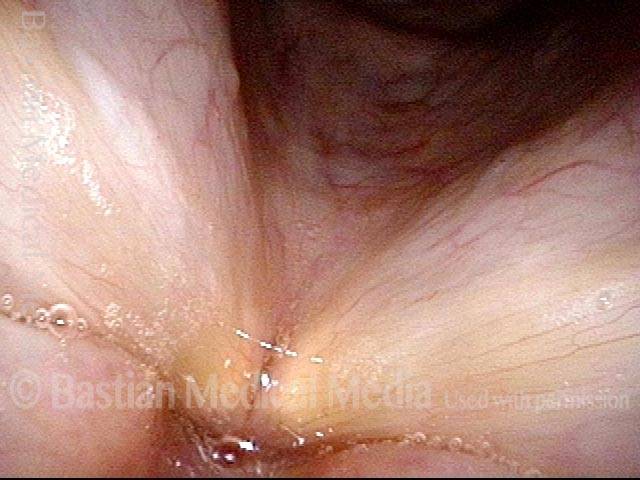
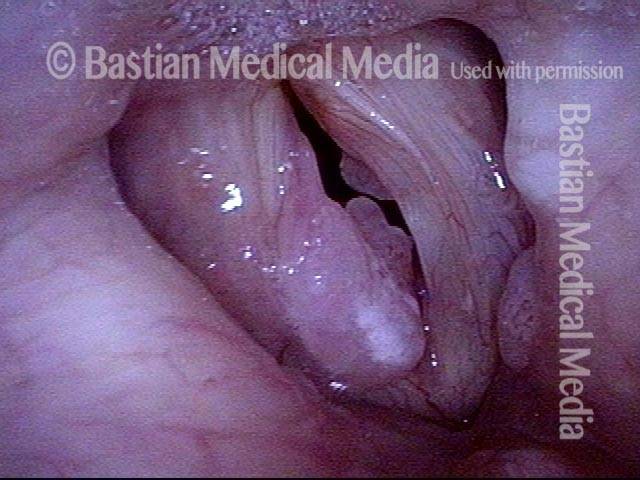
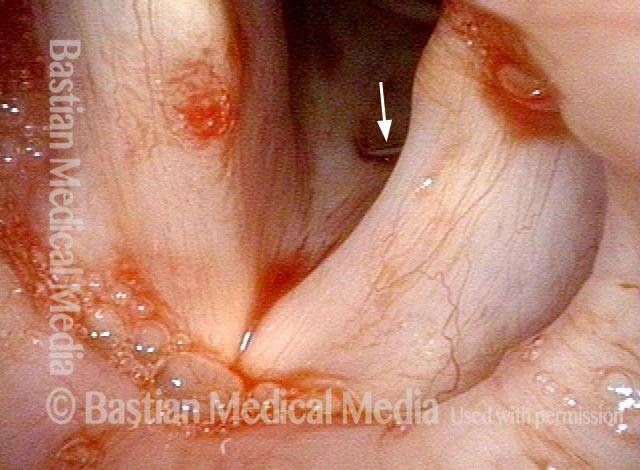
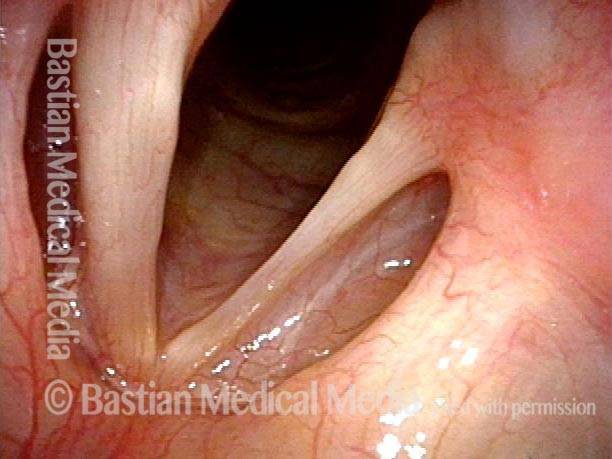
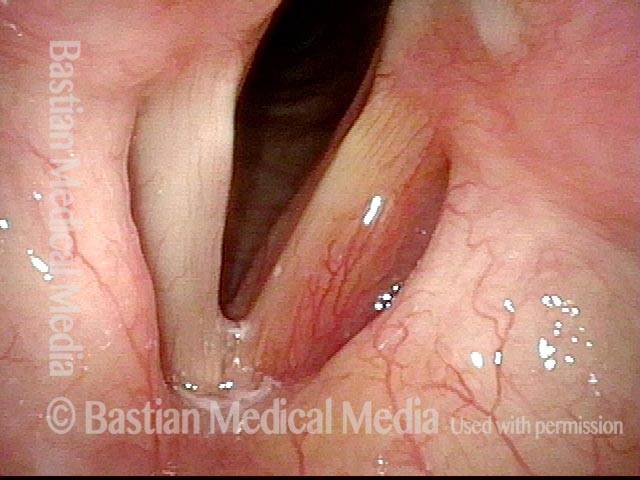
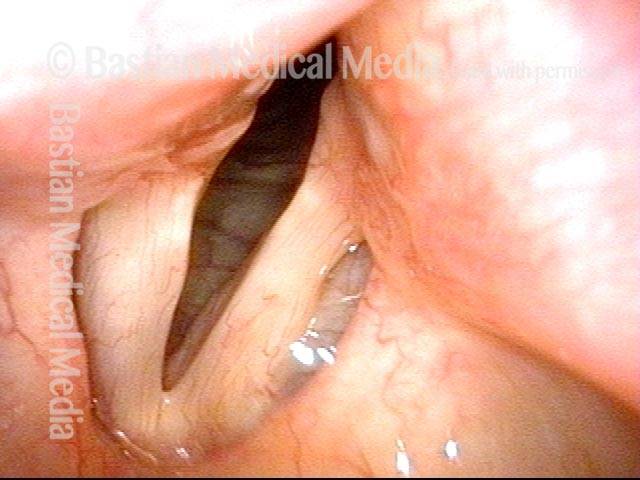
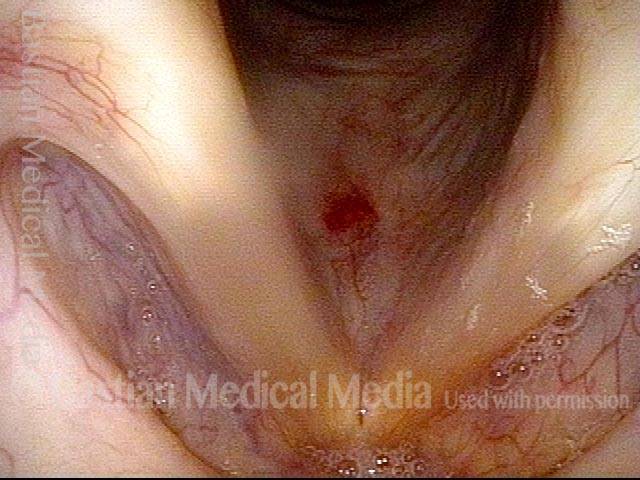
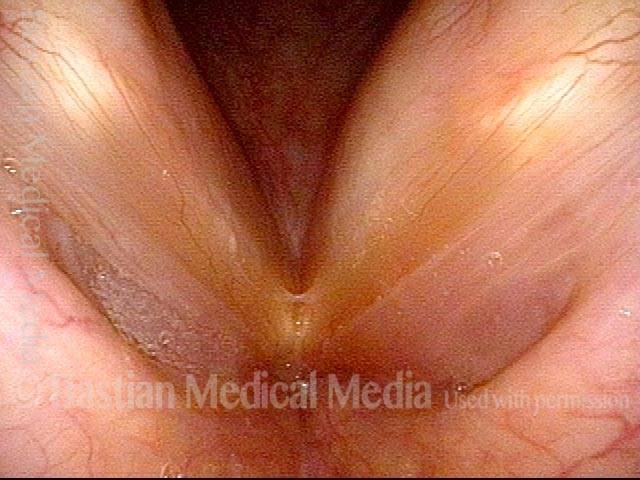
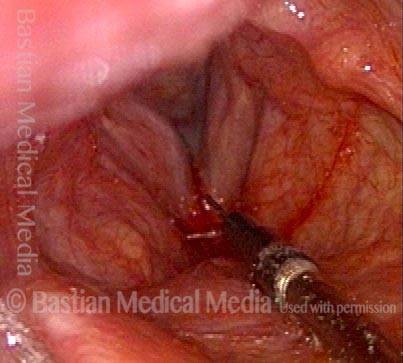
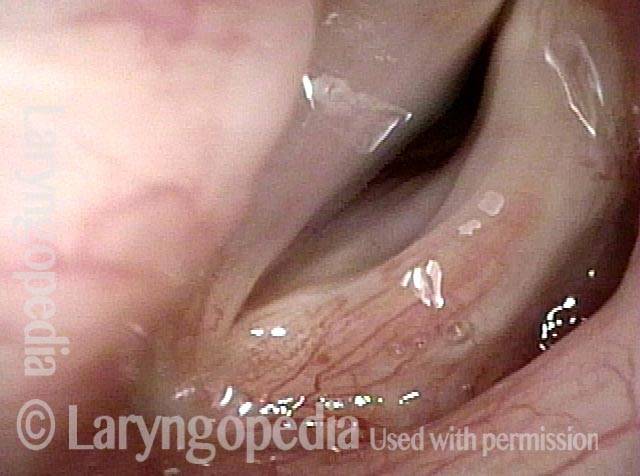
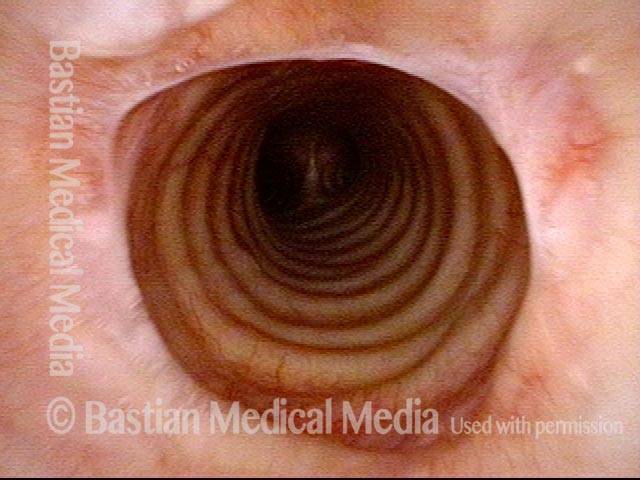
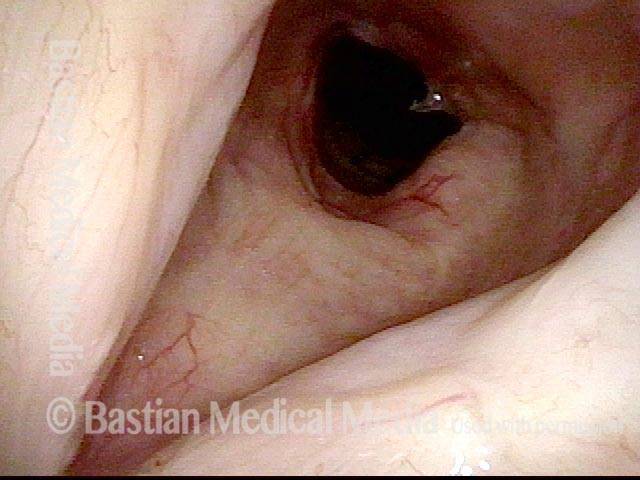
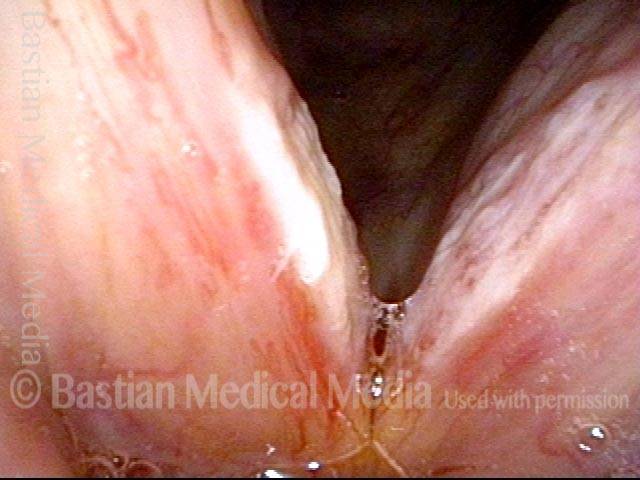
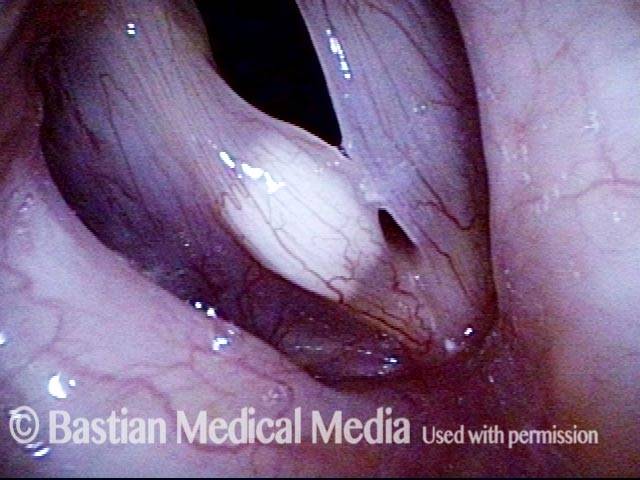
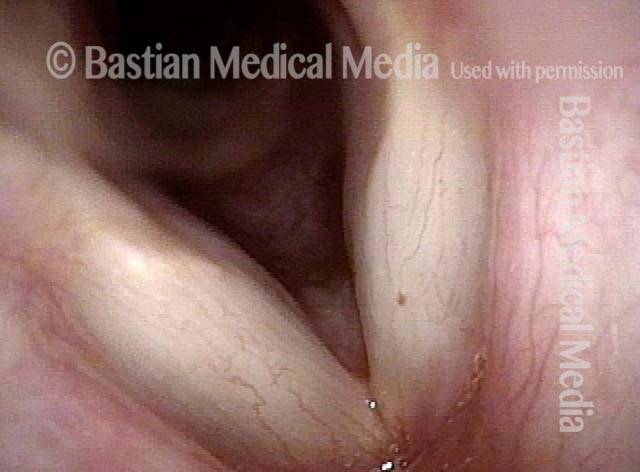
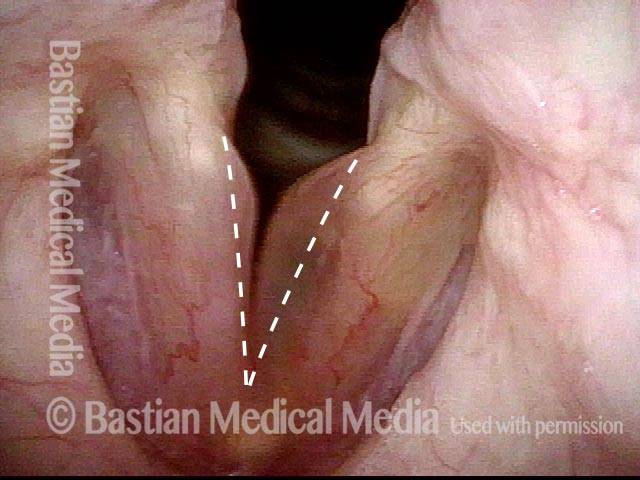
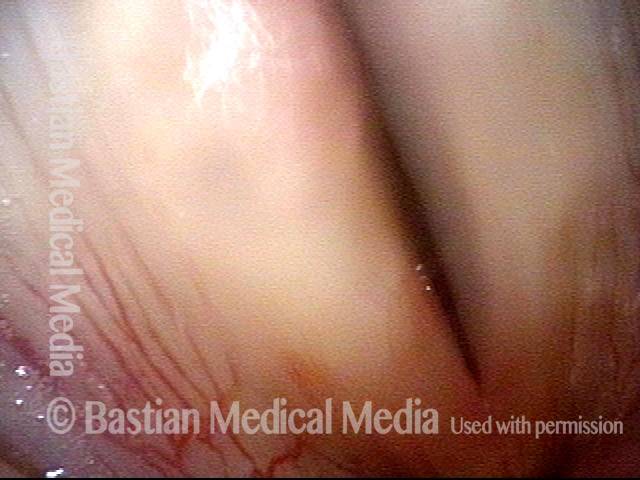
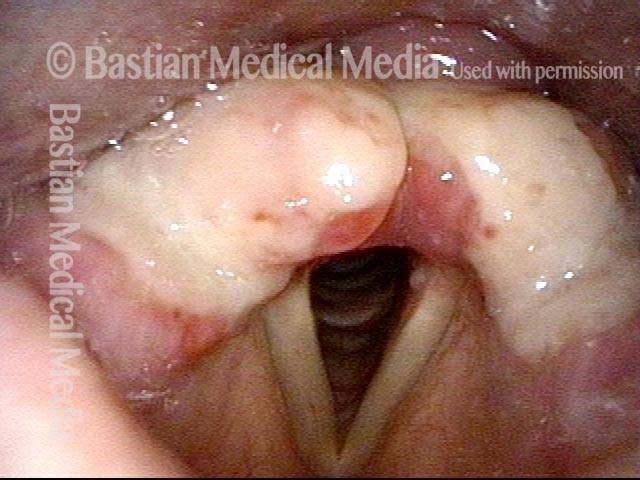
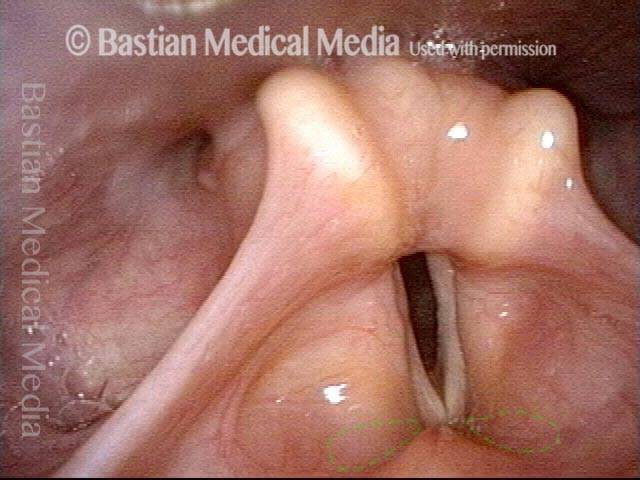
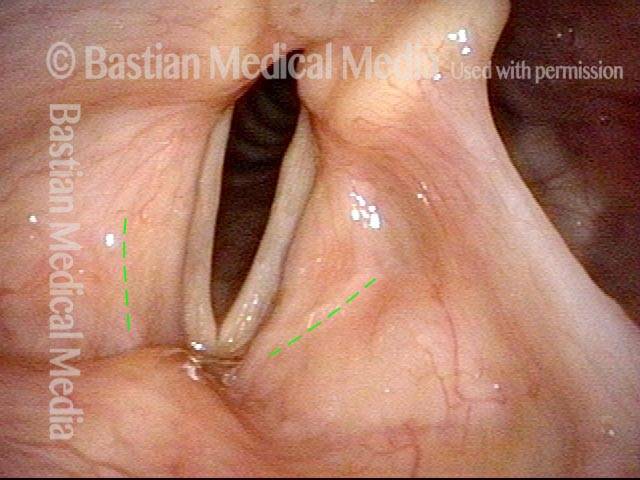
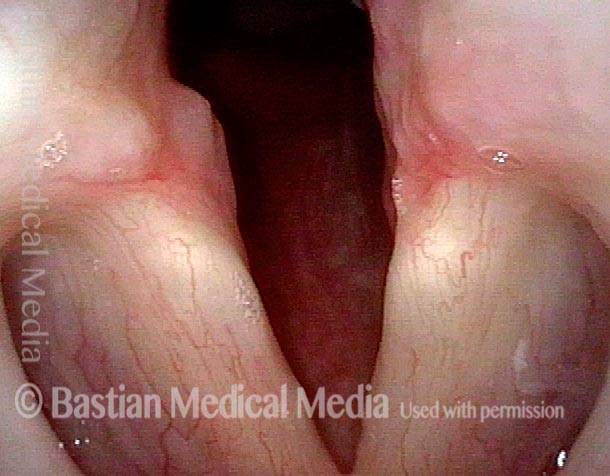
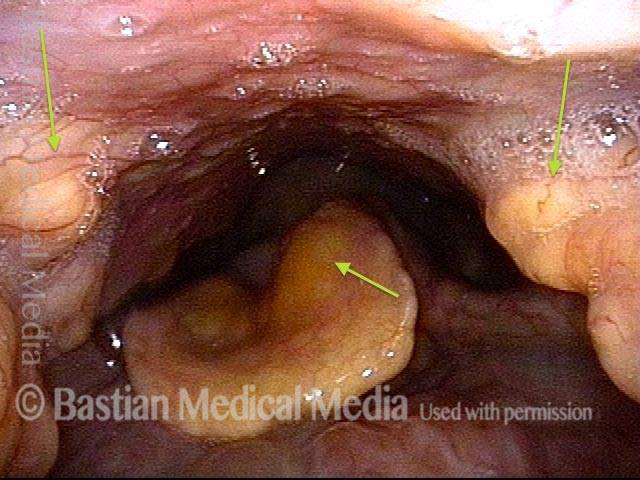
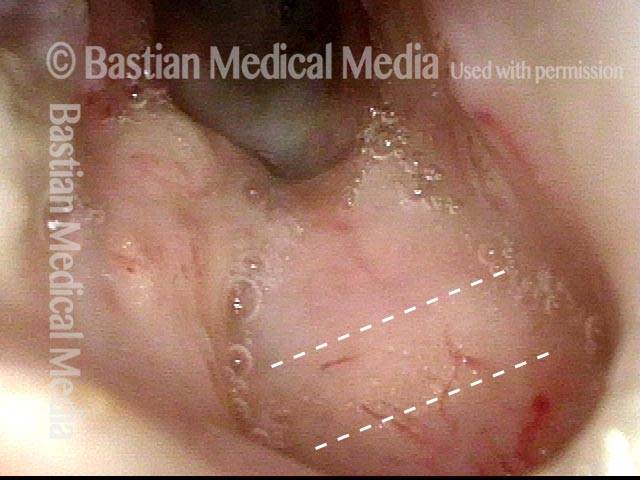
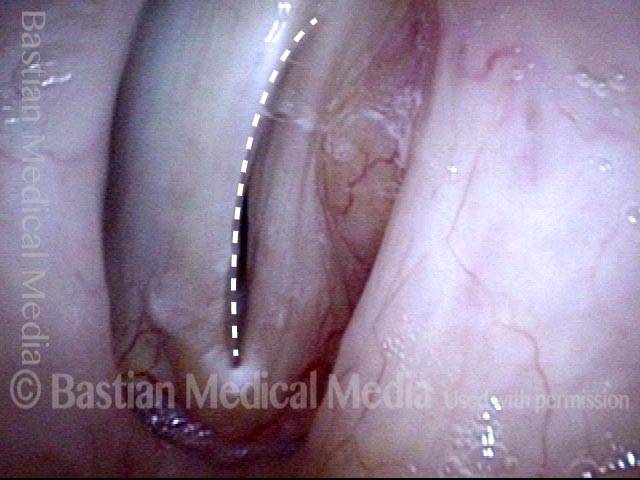
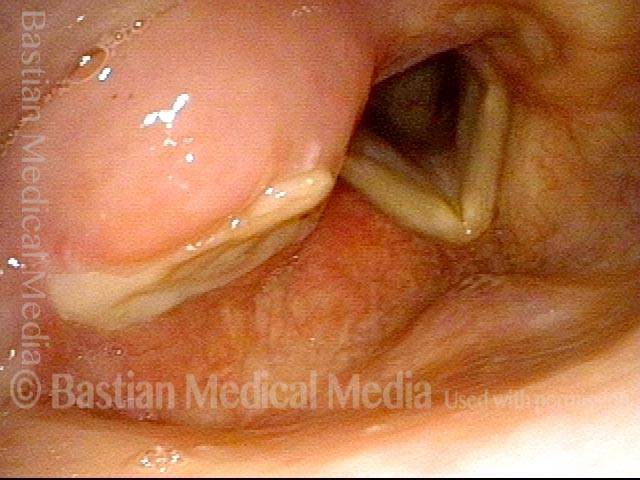
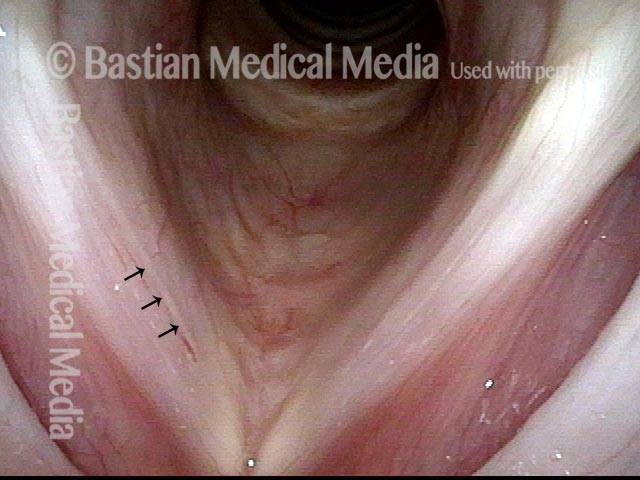
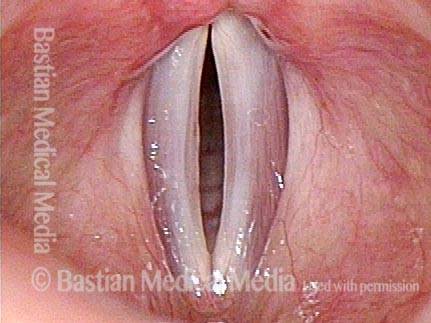
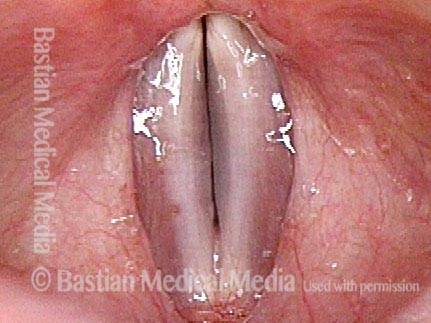
Vocal cord polyp (1 of 8)
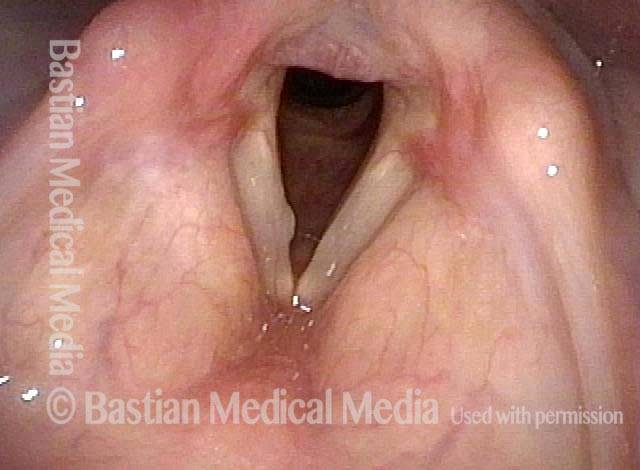
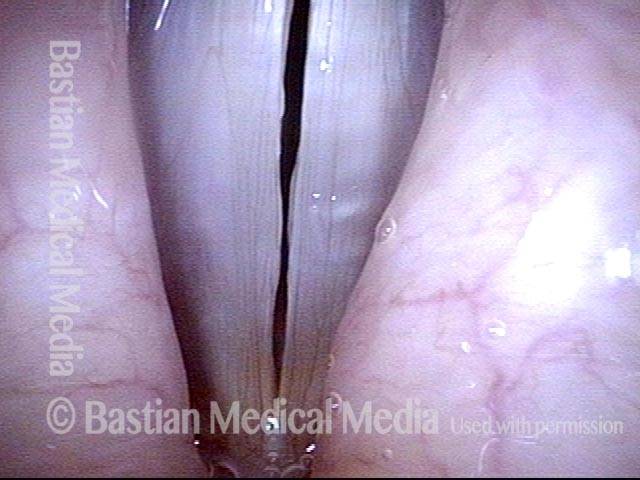
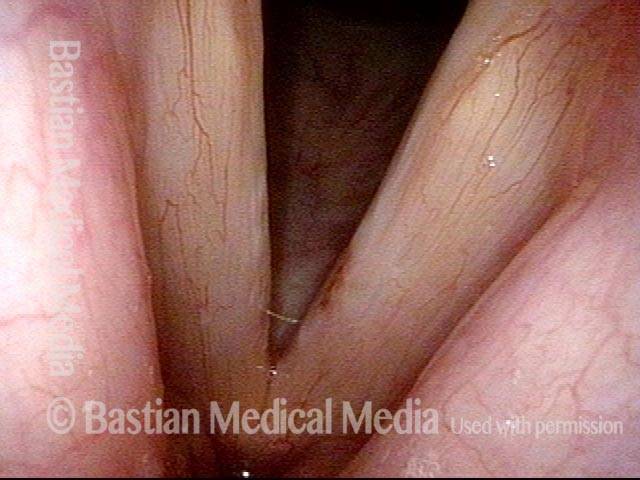
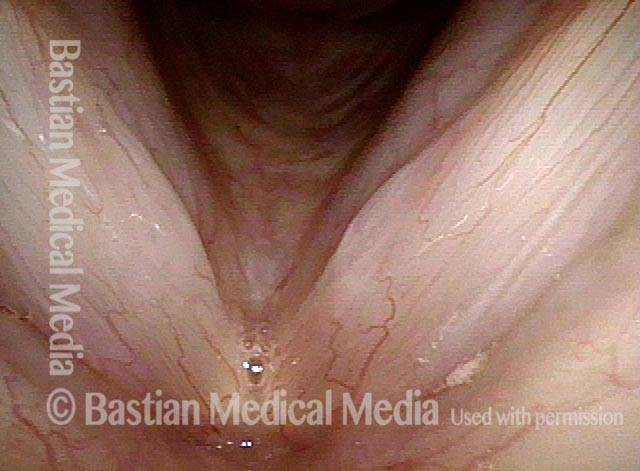
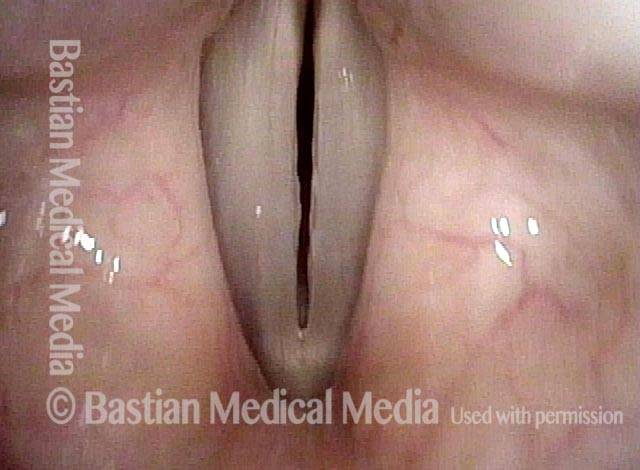
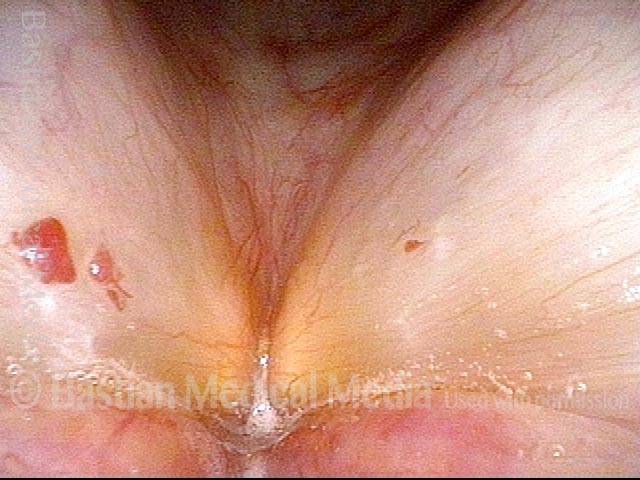
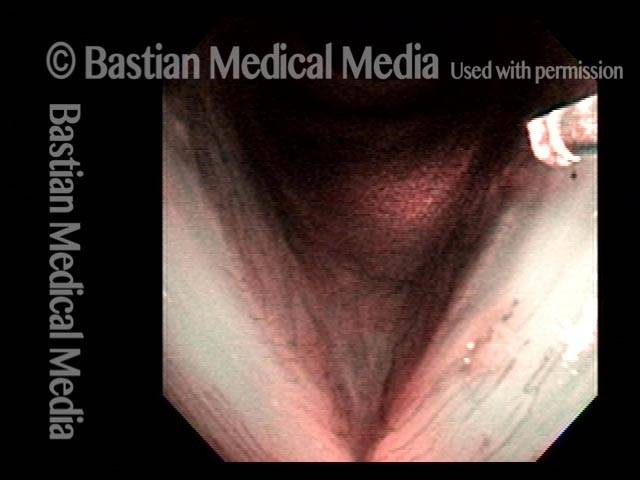
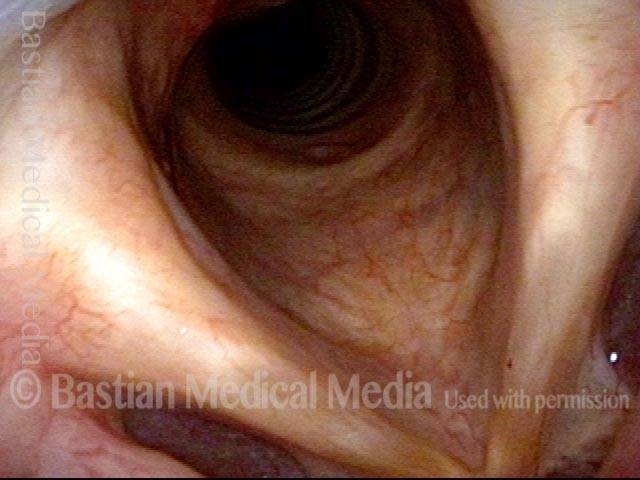
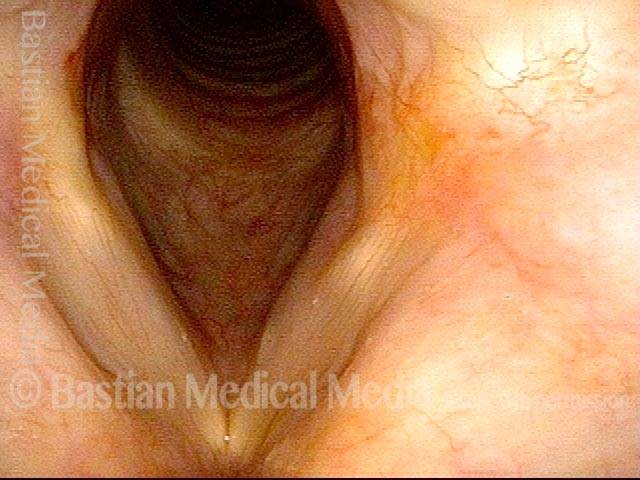
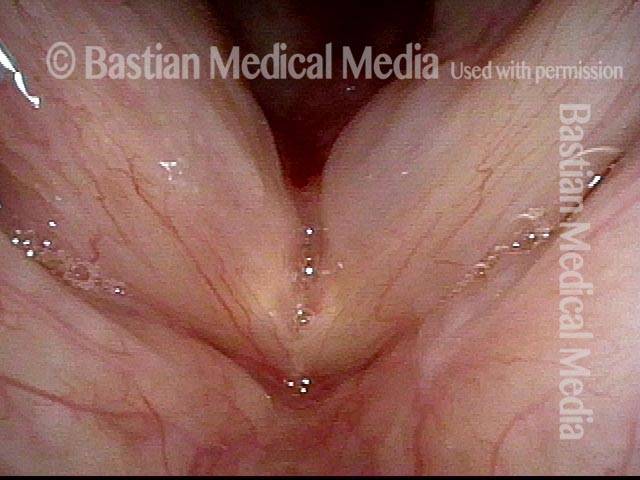
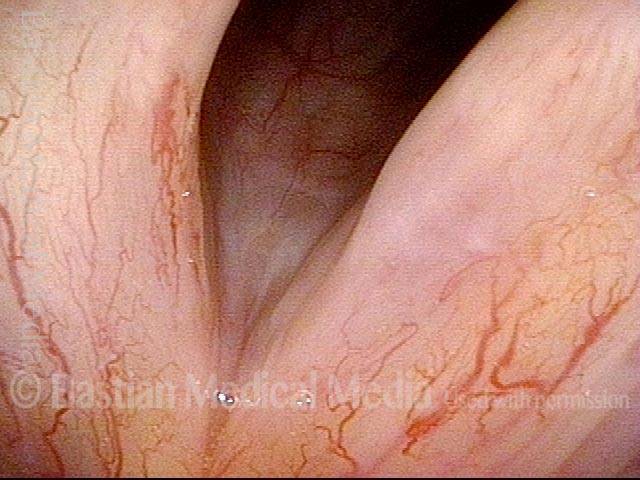
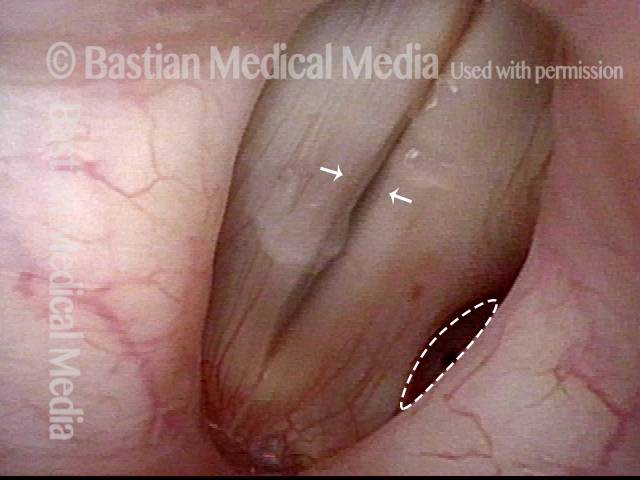
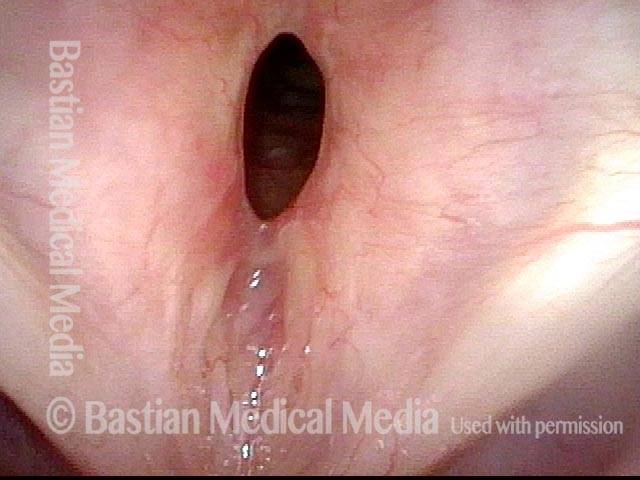
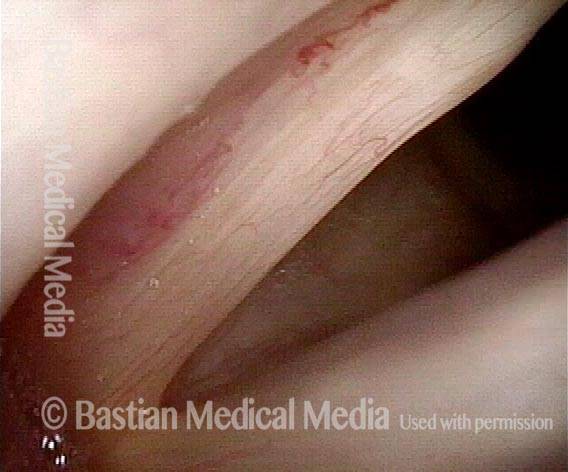
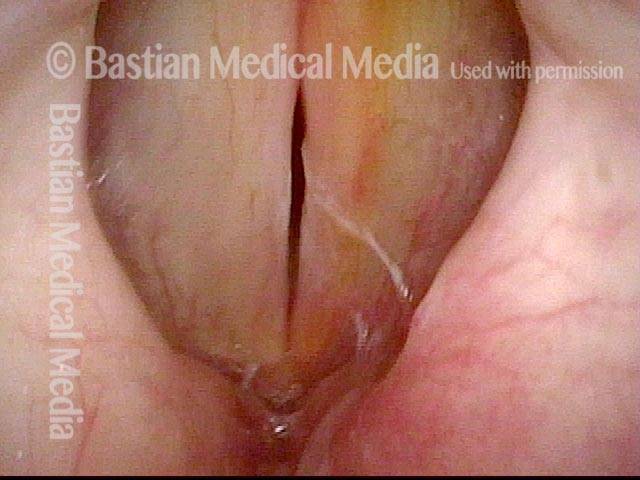
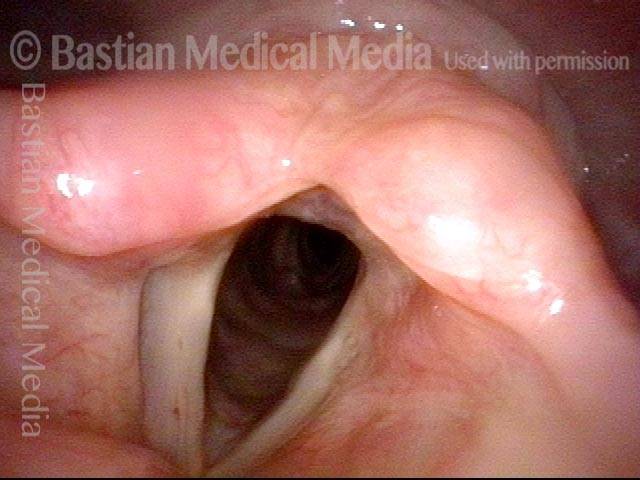
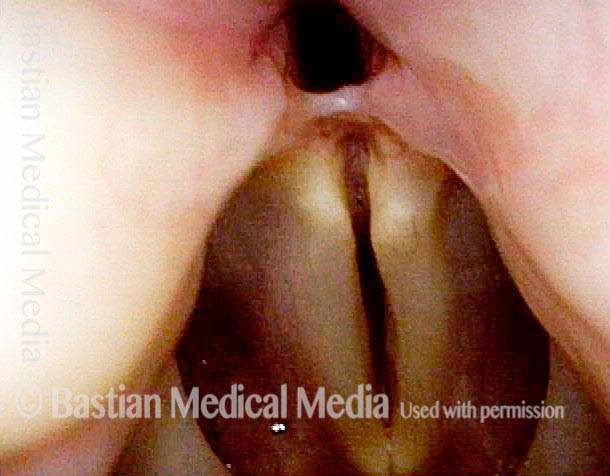
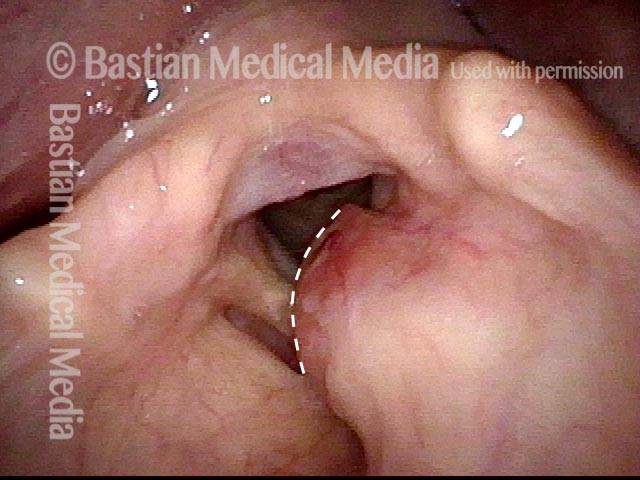
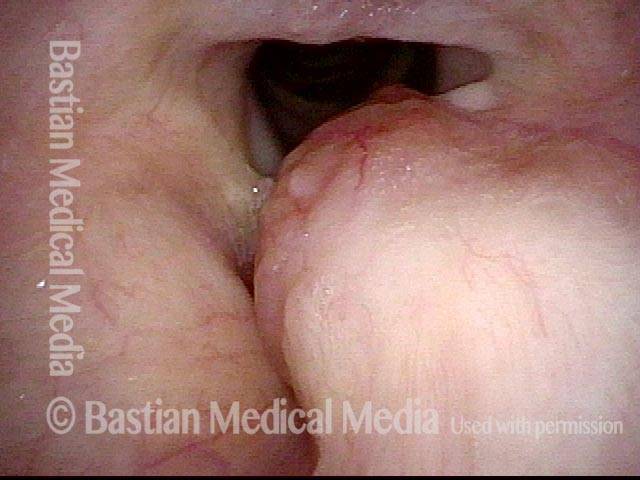
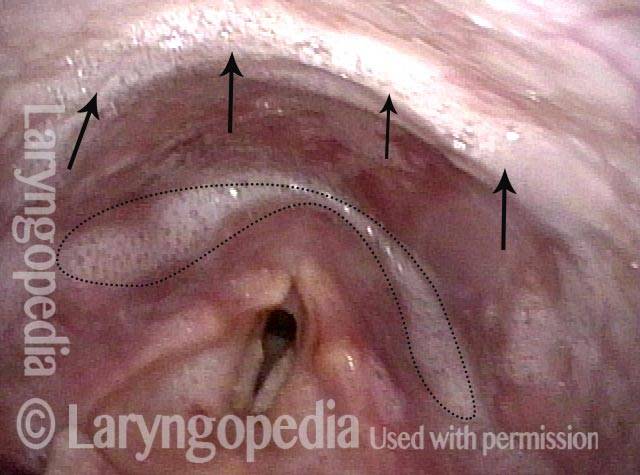
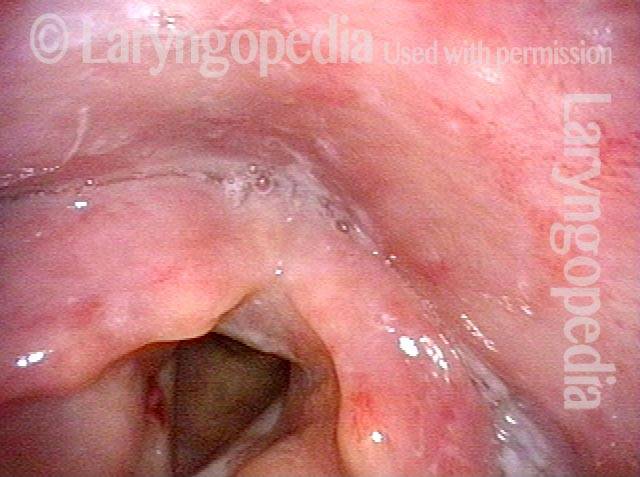
Musical theater actress with chronic hoarseness due to this right vocal cord polyp (left of photo), first identified a year earlier and unresponsive to speech therapy.
Vocal cord polyp (1 of 8)
Musical theater actress with chronic hoarseness due to this right vocal cord polyp (left of photo), first identified a year earlier and unresponsive to speech therapy.
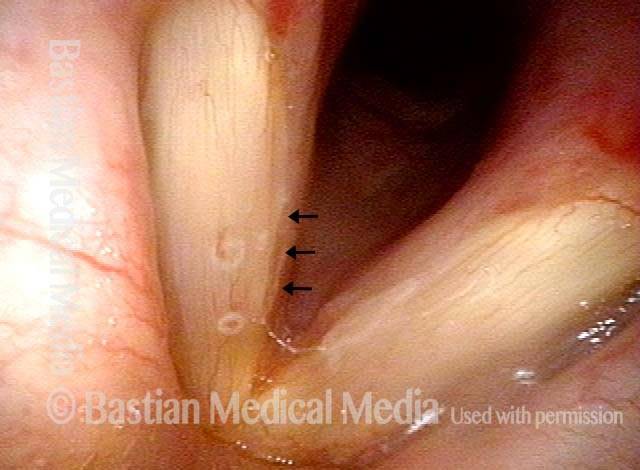
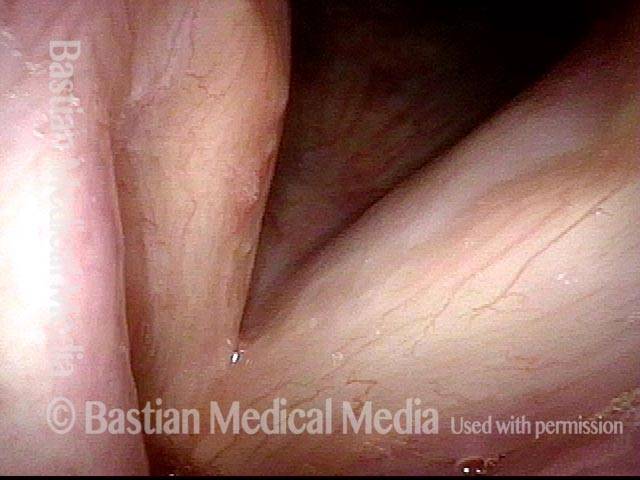
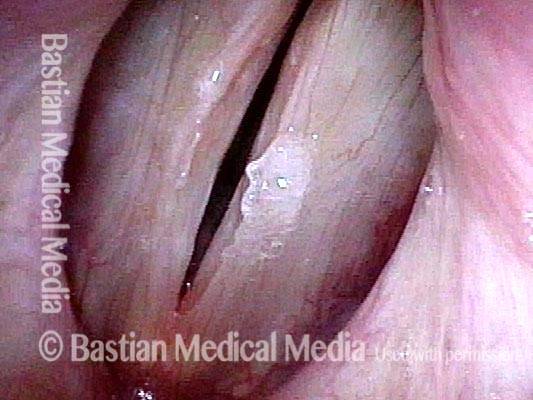
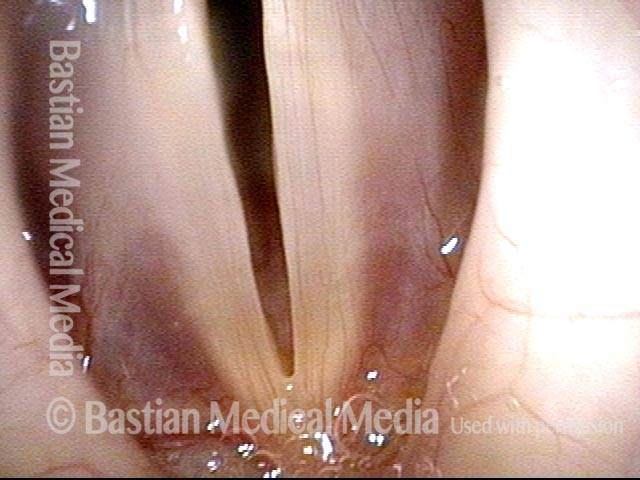
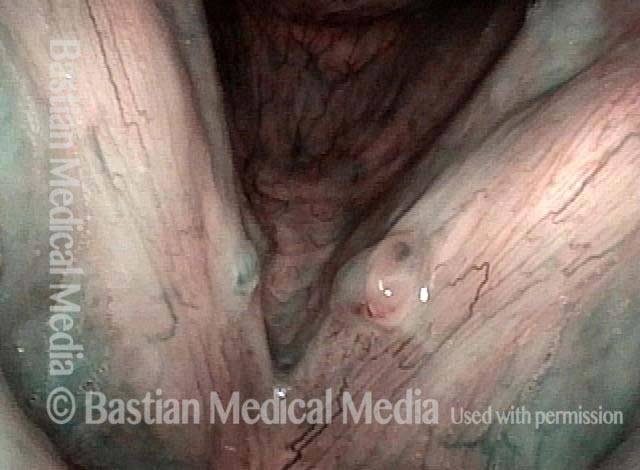
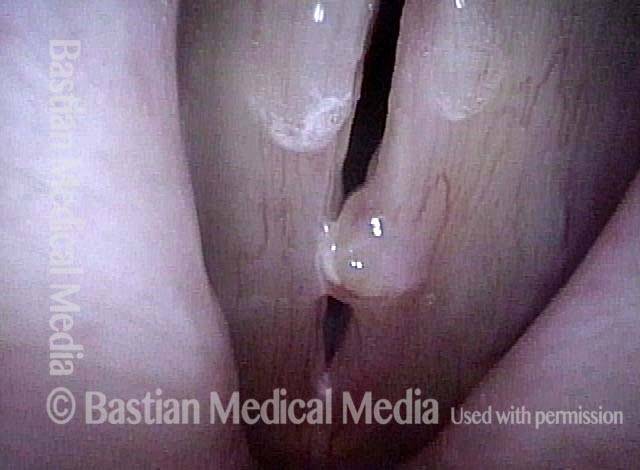
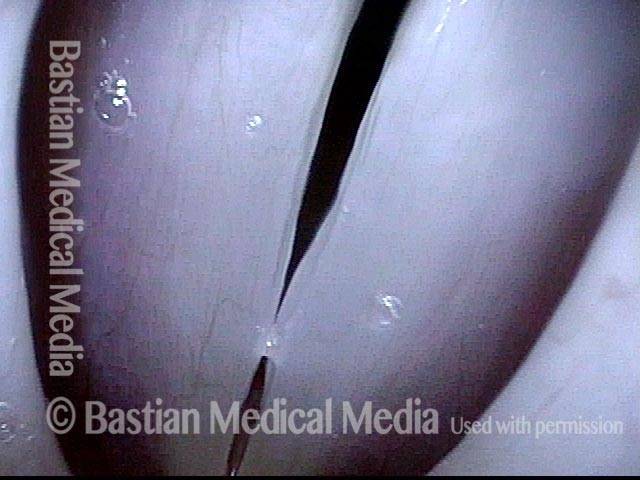
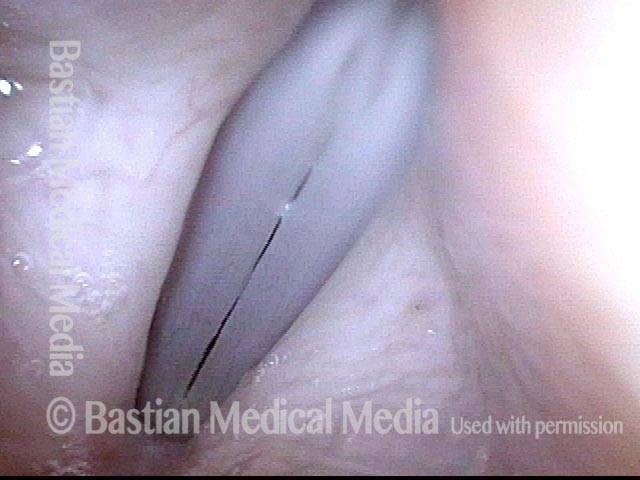
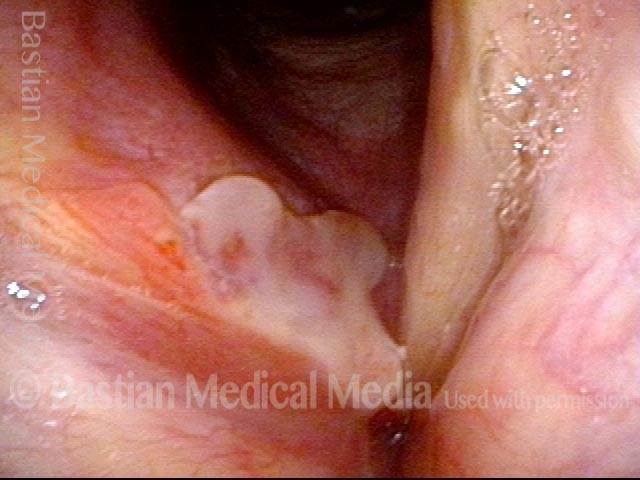
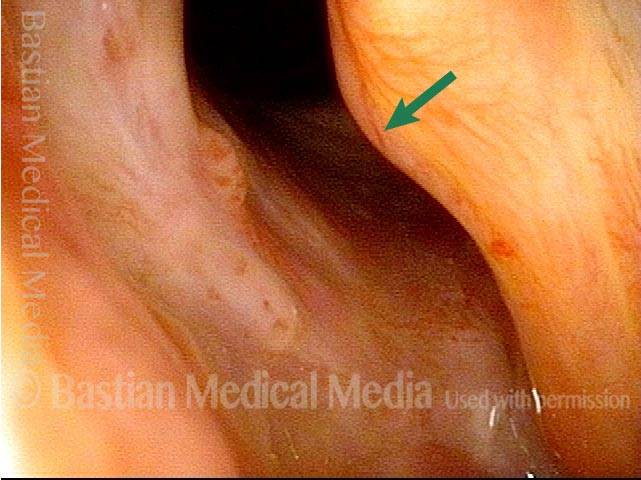
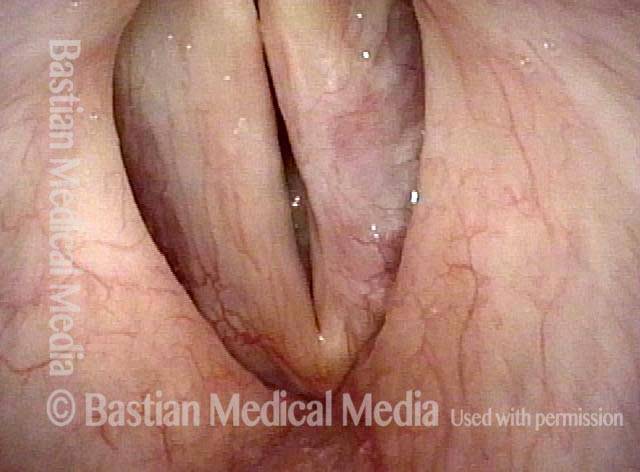
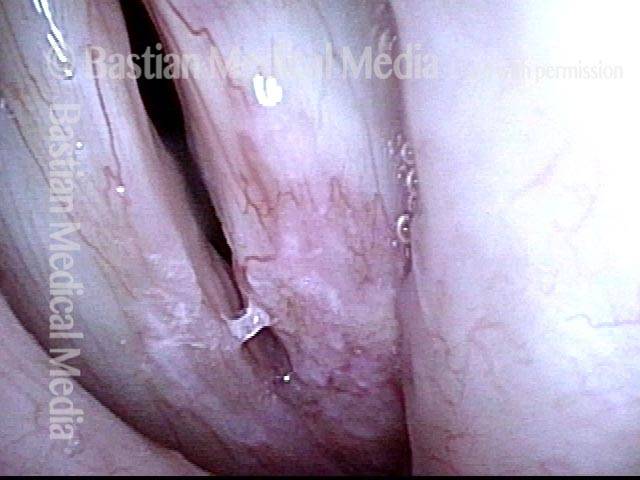
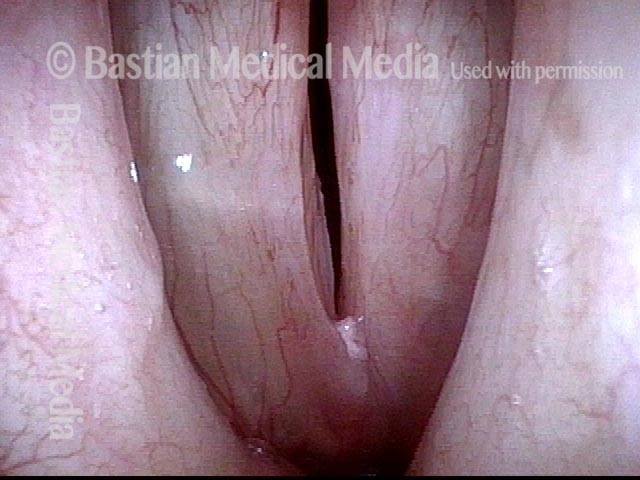
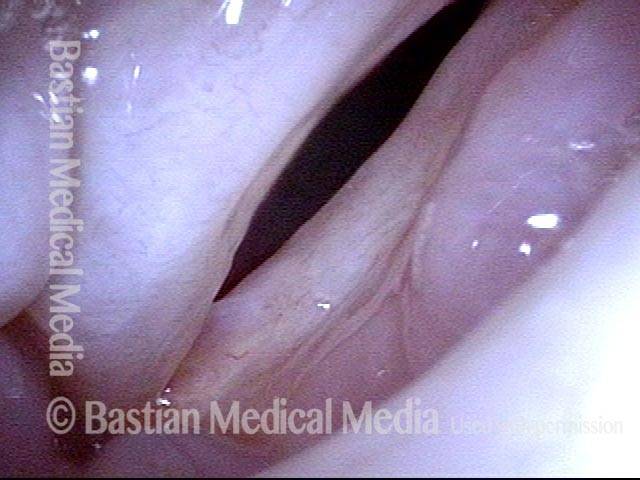
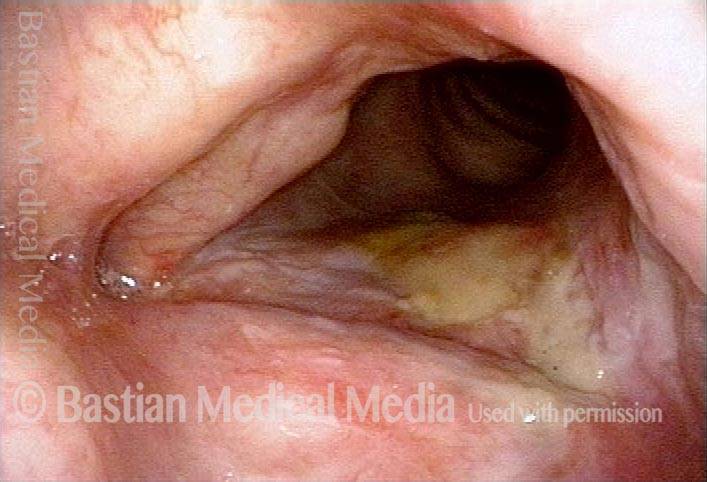
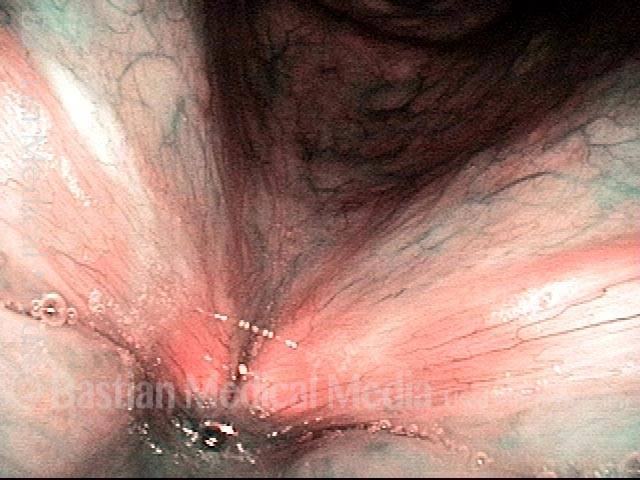
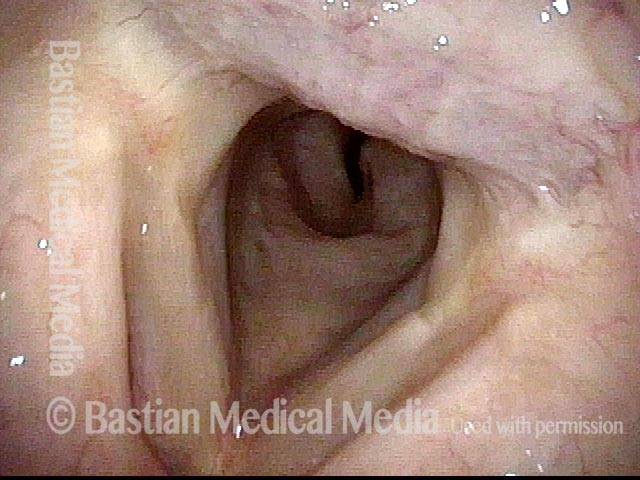
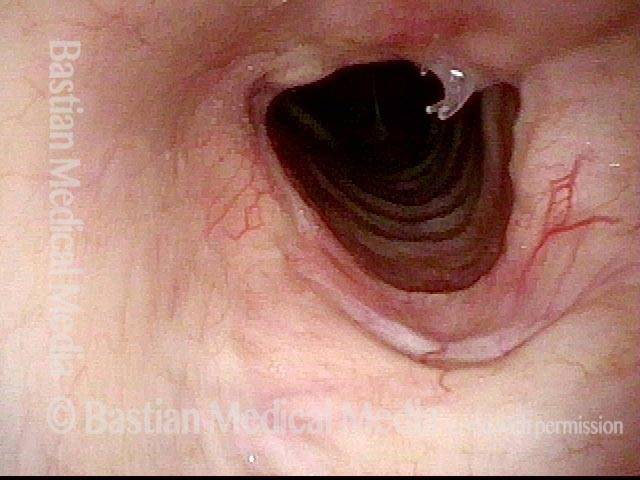
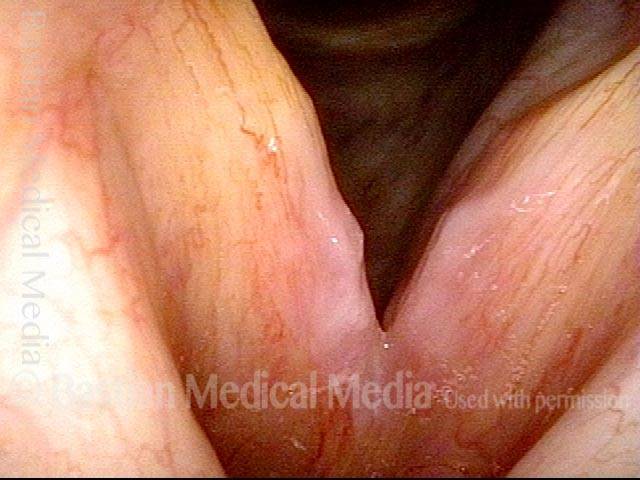
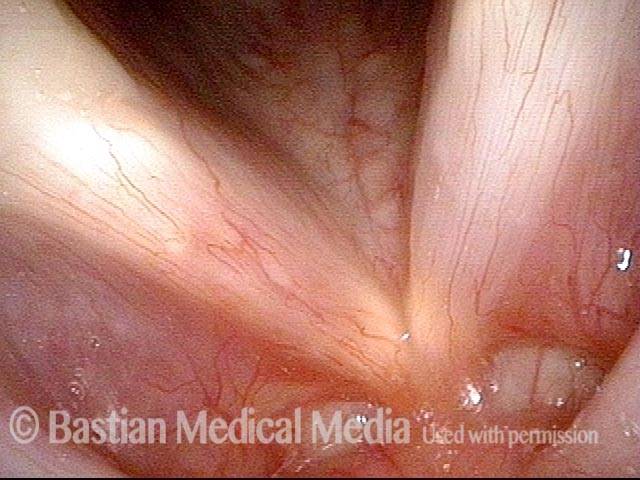
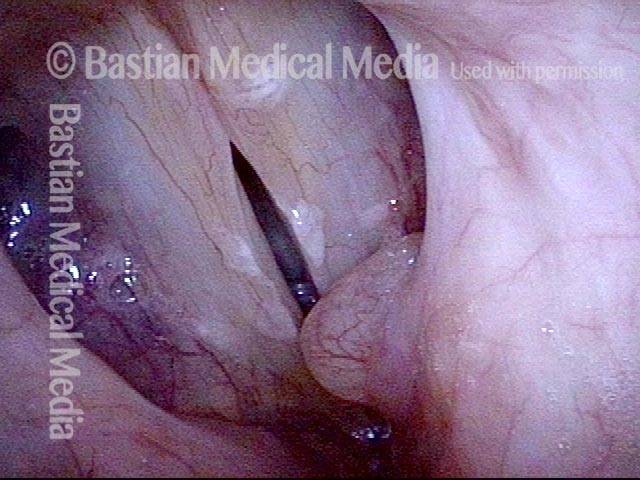
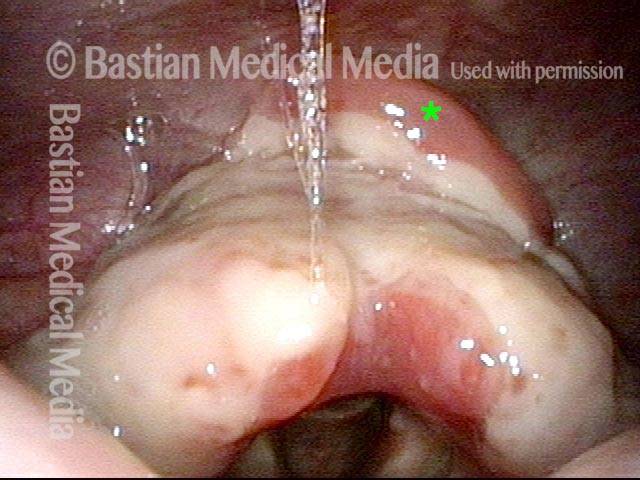
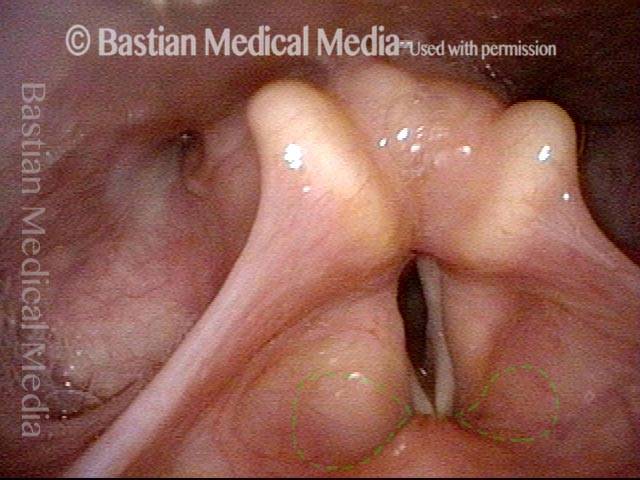
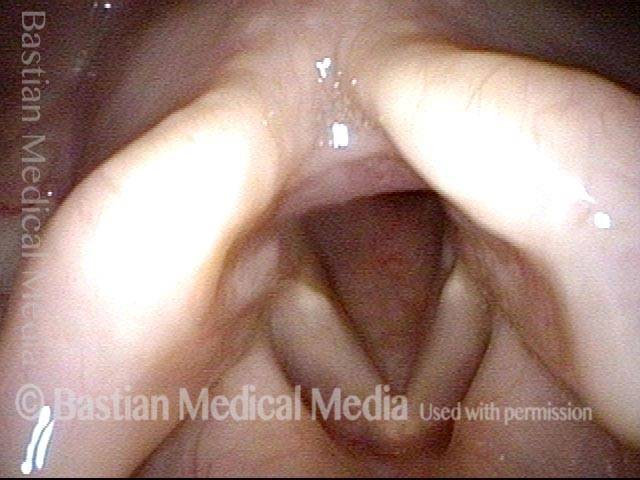
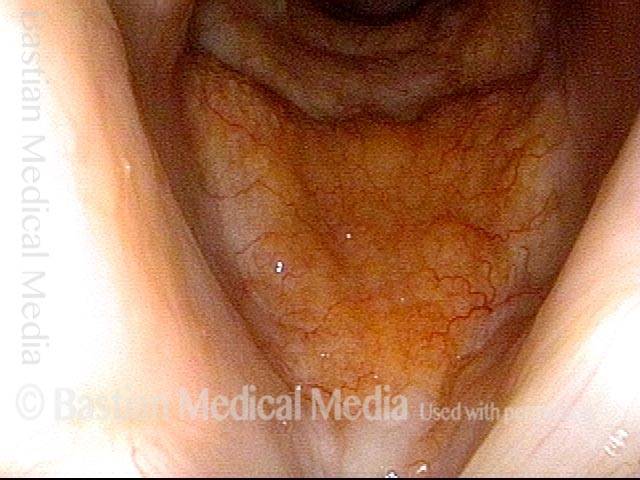
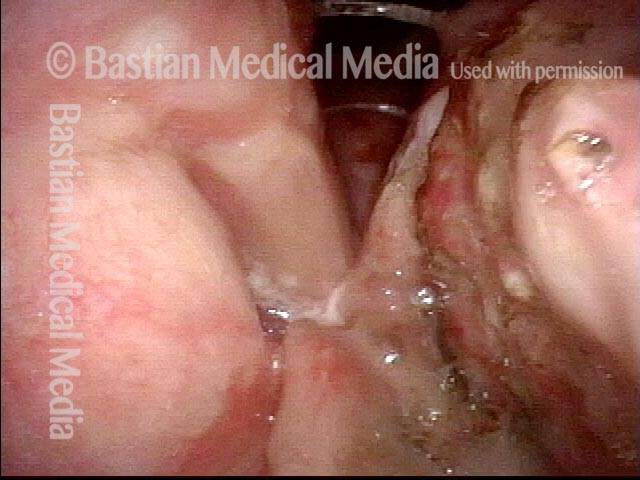
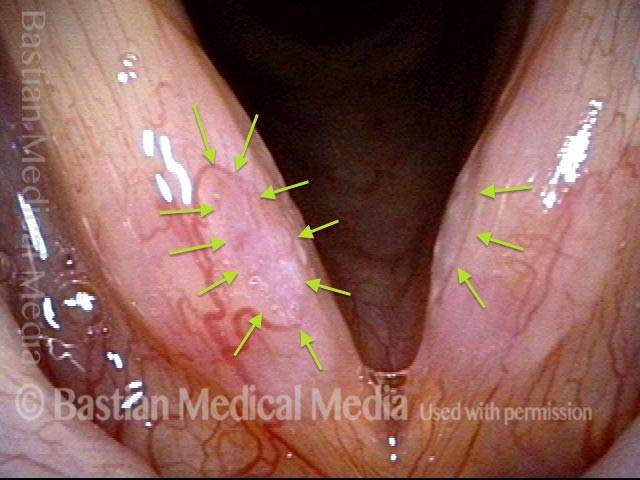
Closer view (2 of 8)
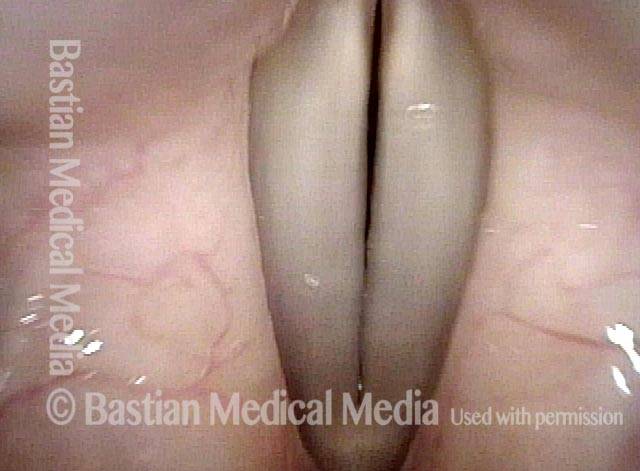
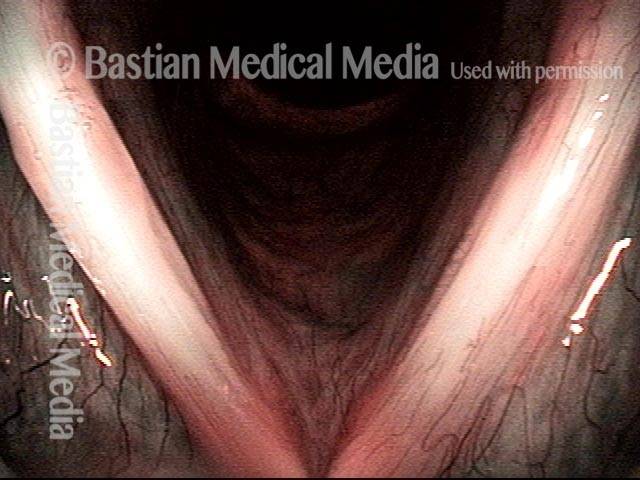
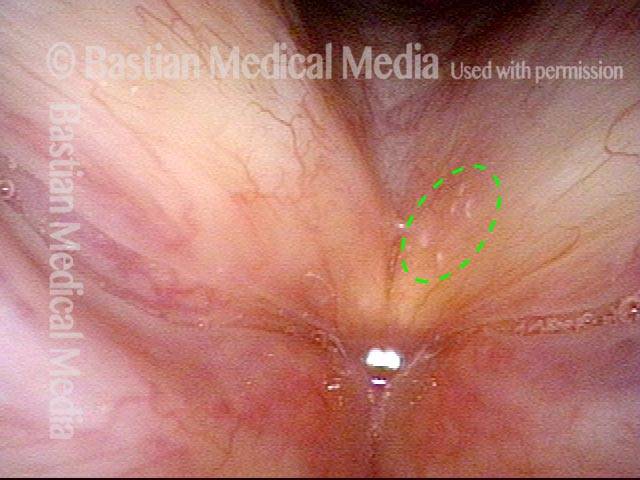
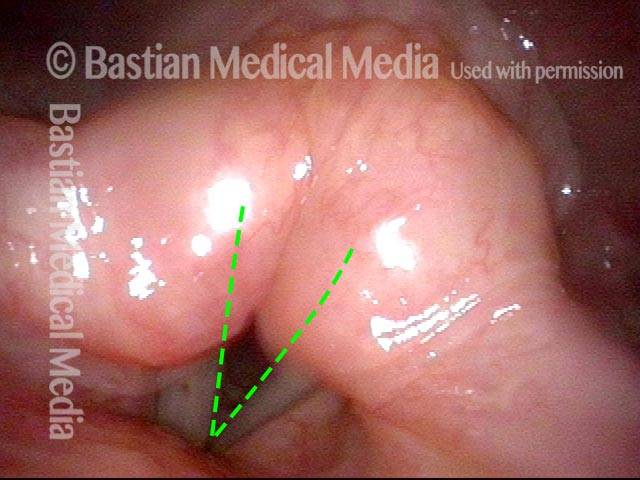
Magnified view shows small elevation of the left cord (right of photo) as well.
Closer view (2 of 8)
Magnified view shows small elevation of the left cord (right of photo) as well.
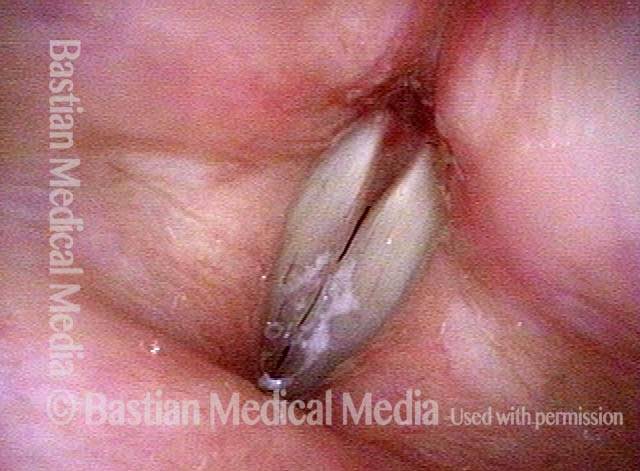
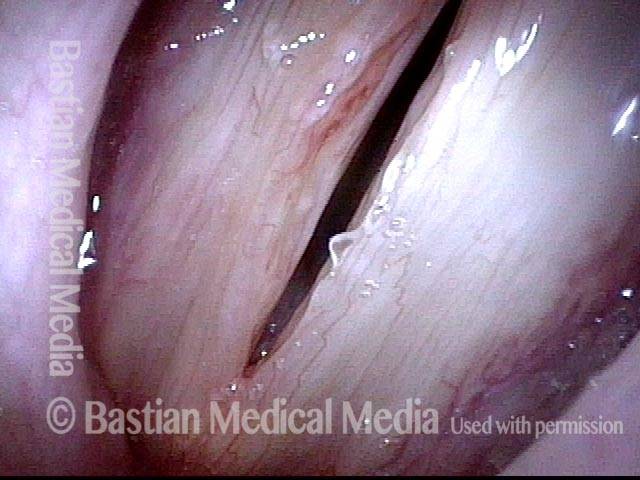
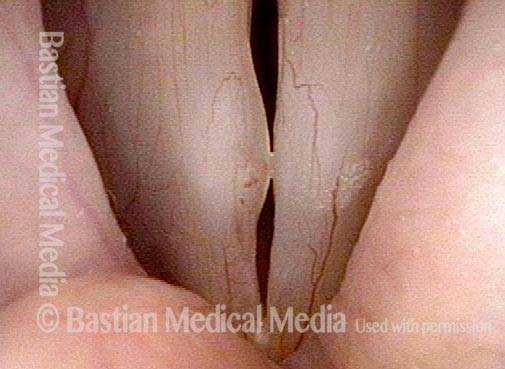
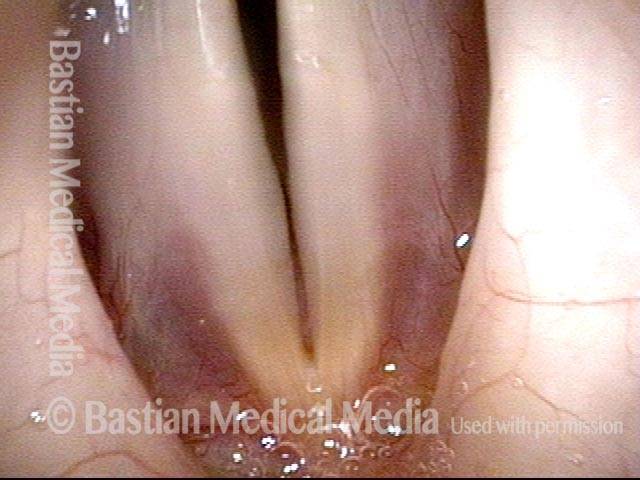
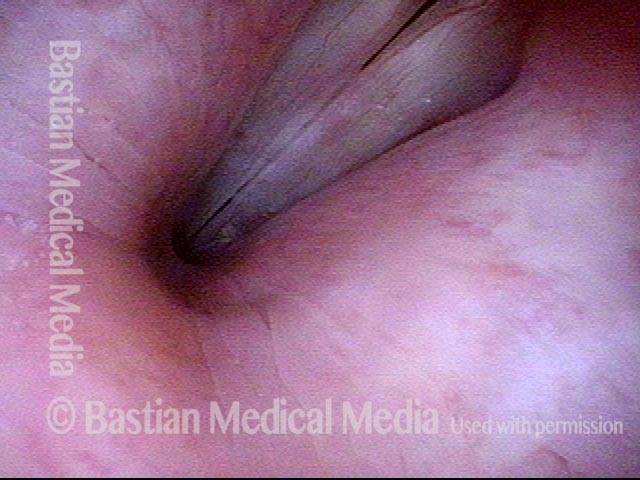
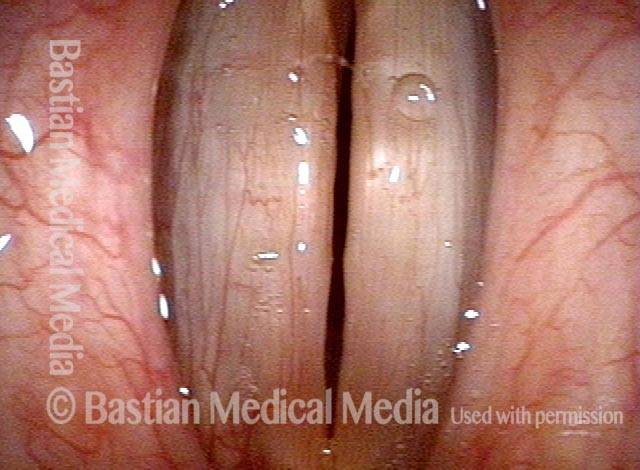
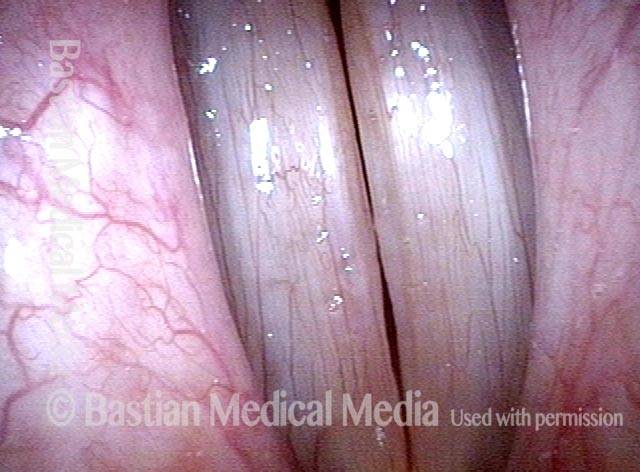
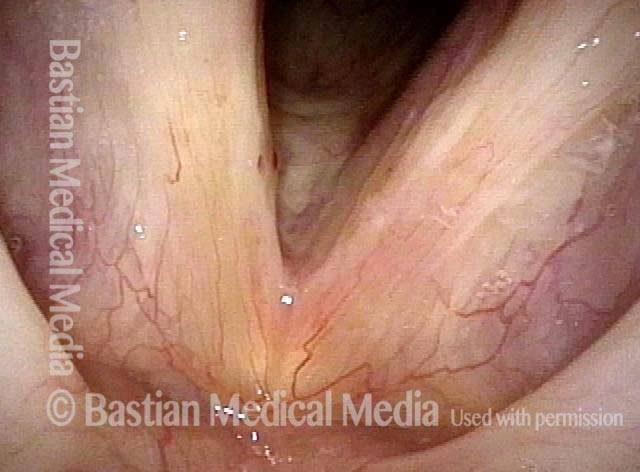
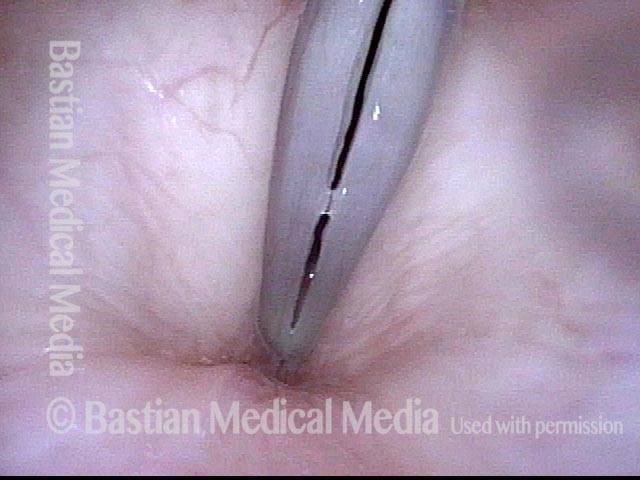
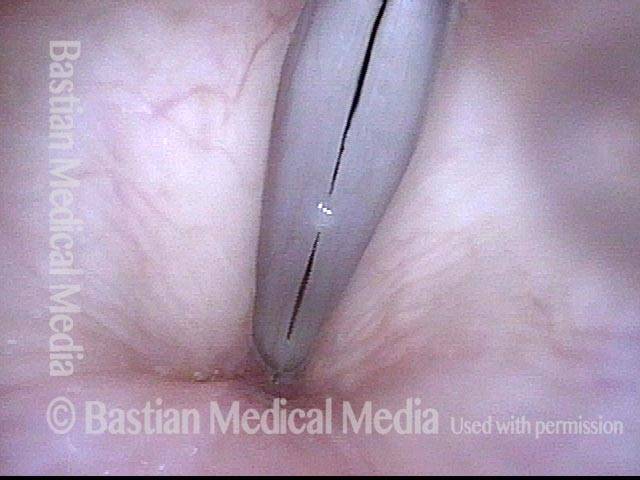
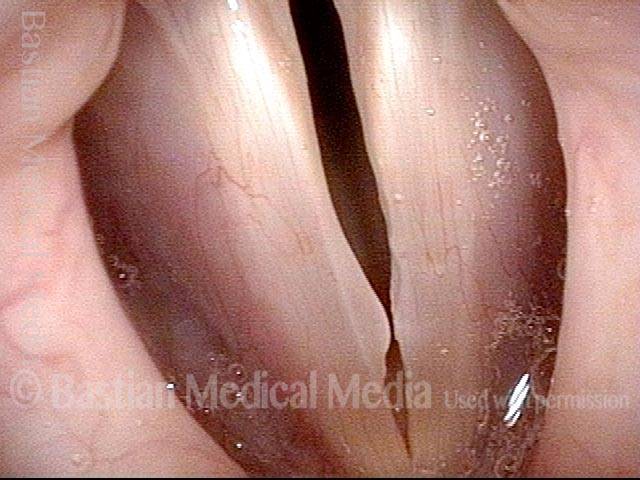
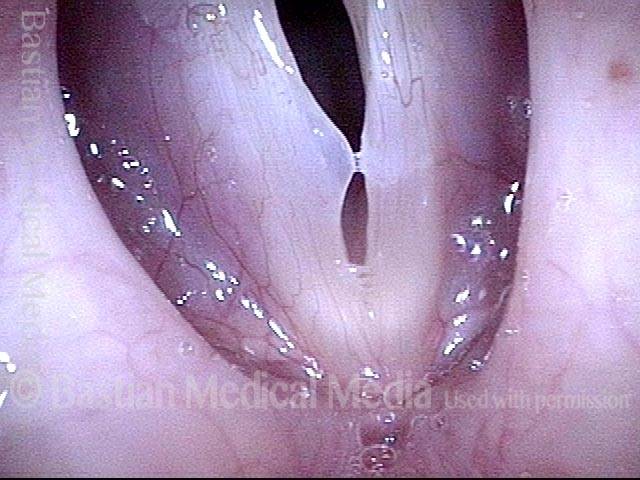
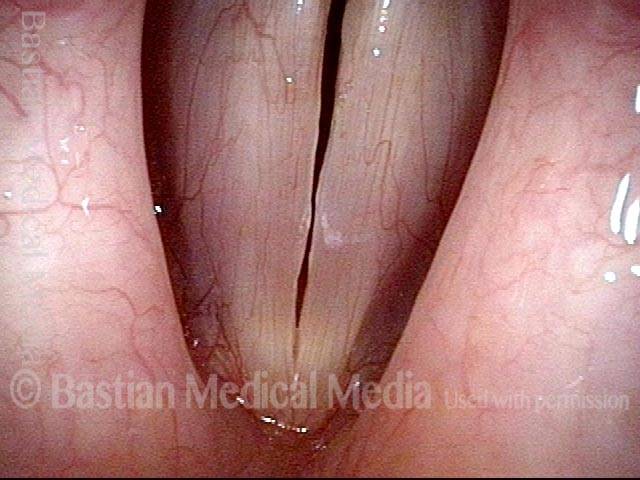
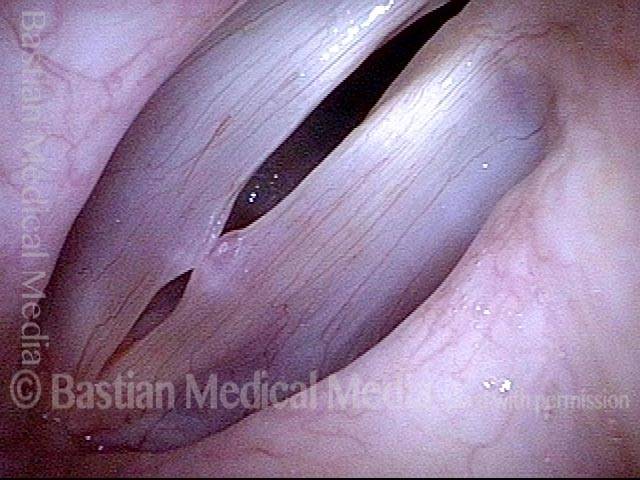
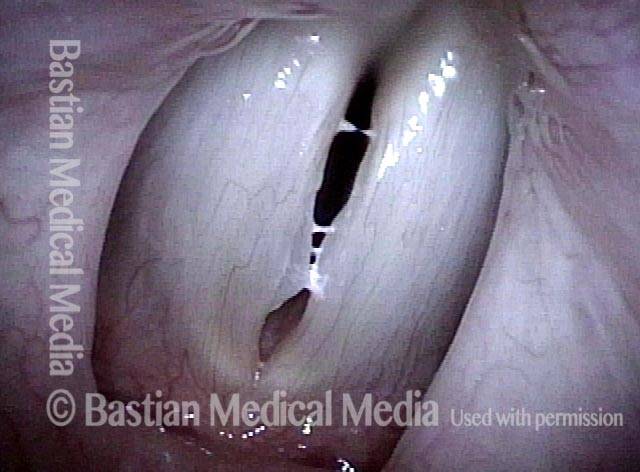
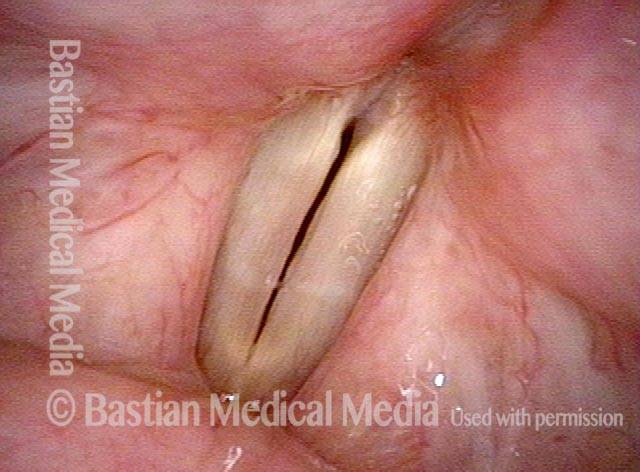
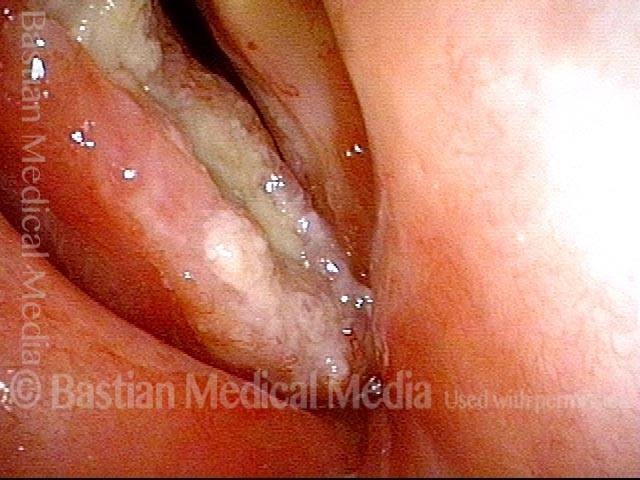
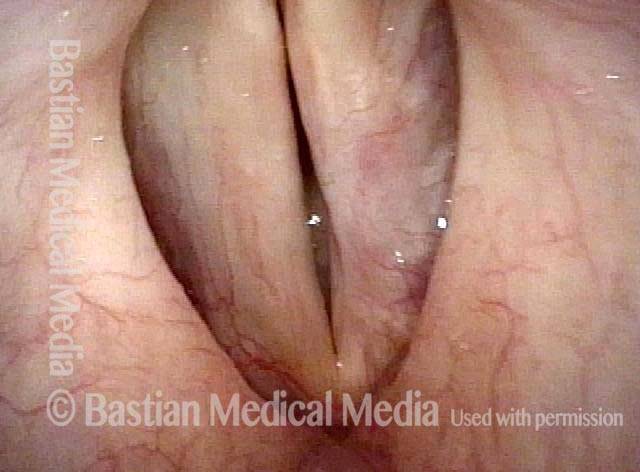
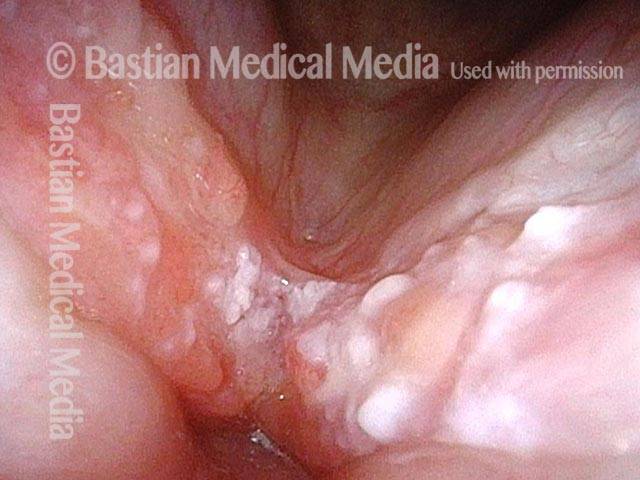
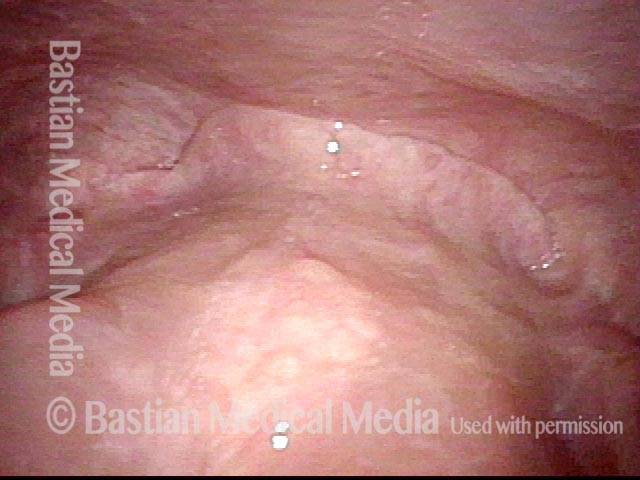
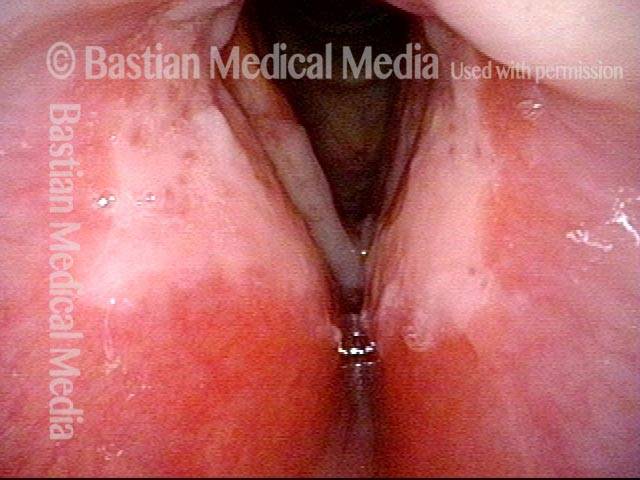
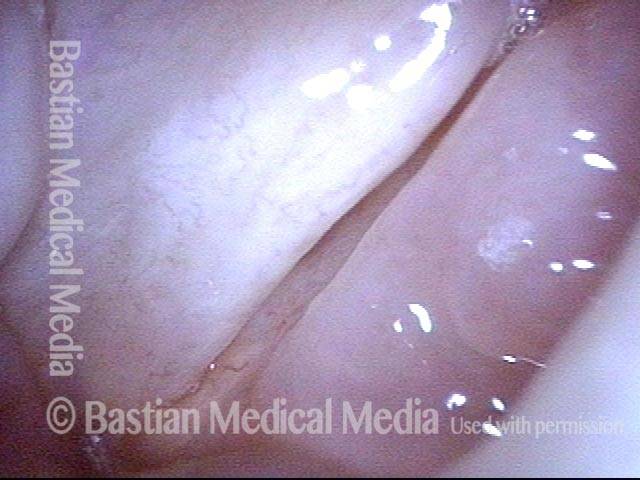
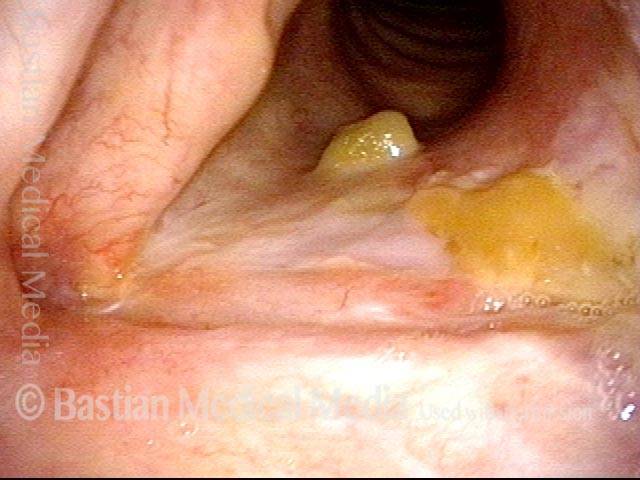
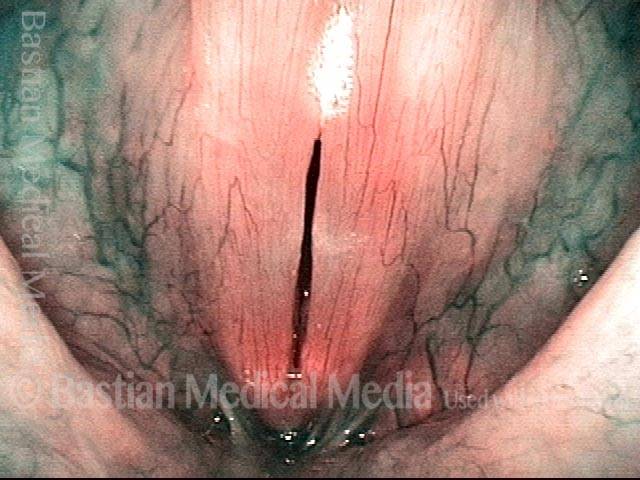
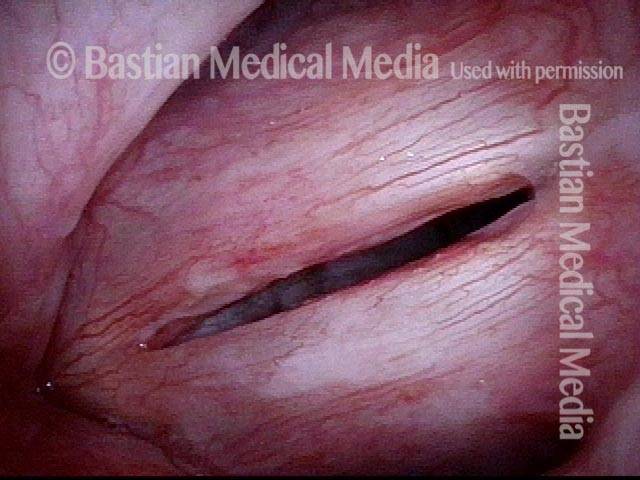
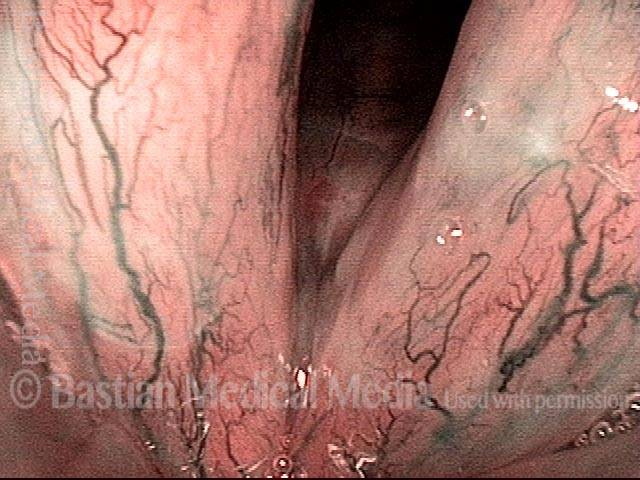
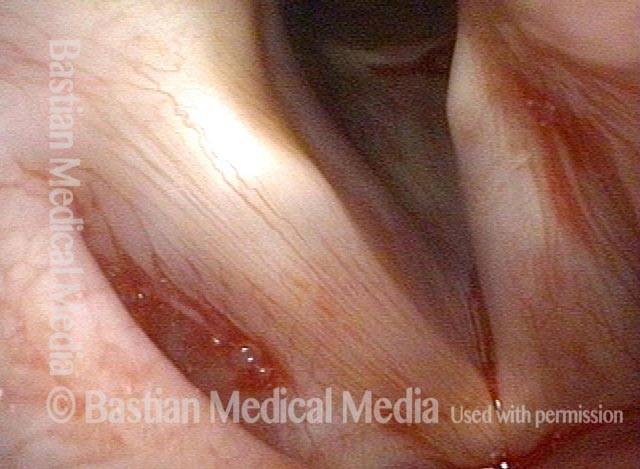
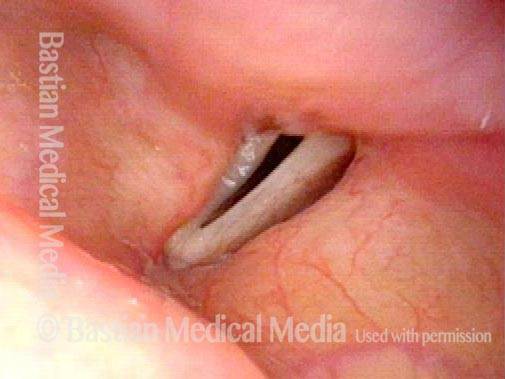
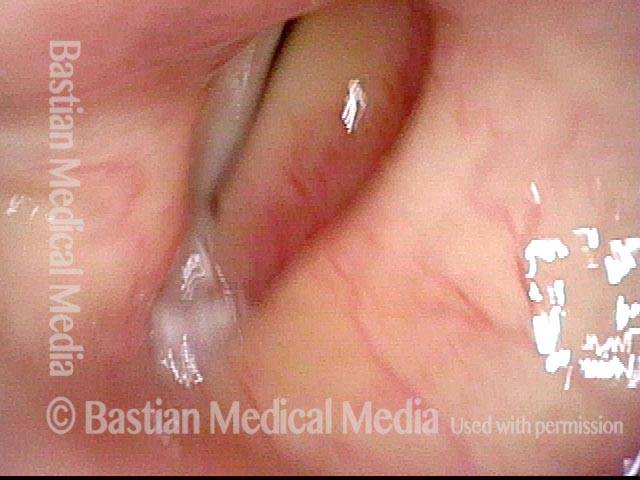
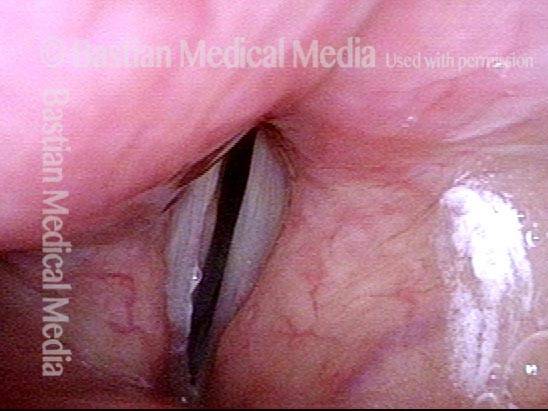
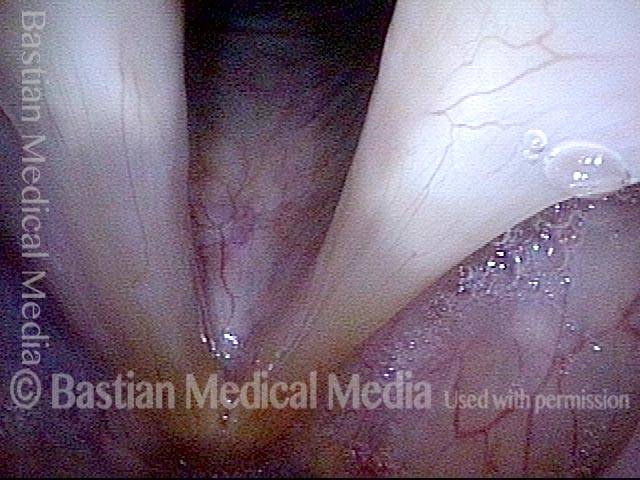
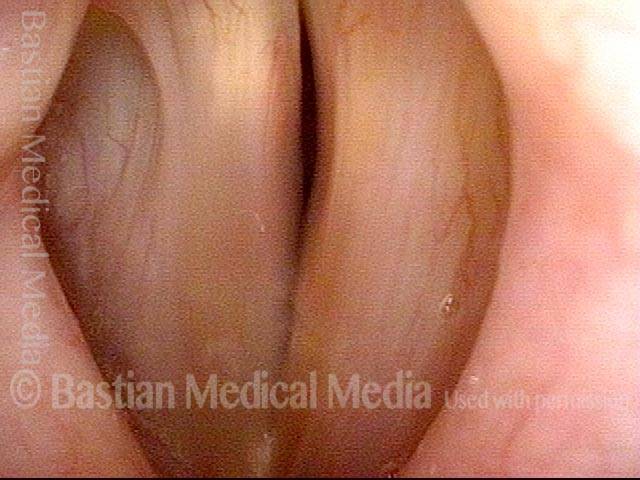
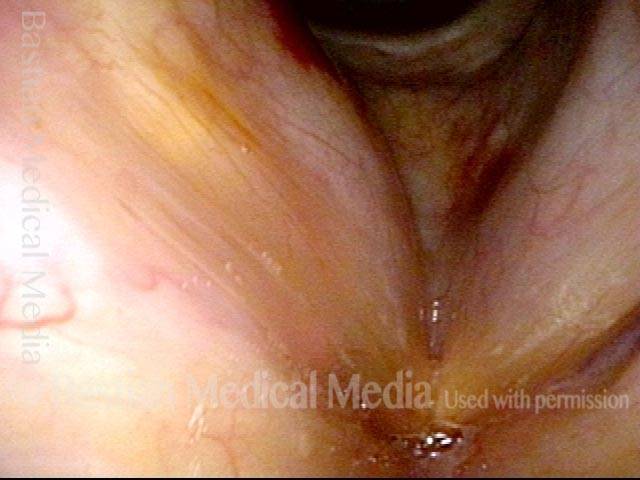
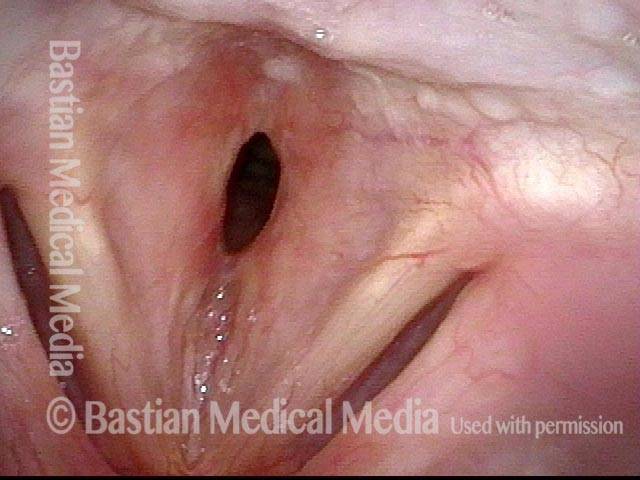
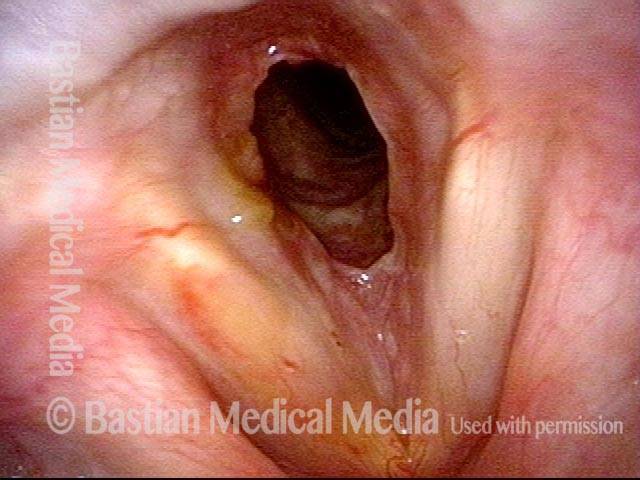
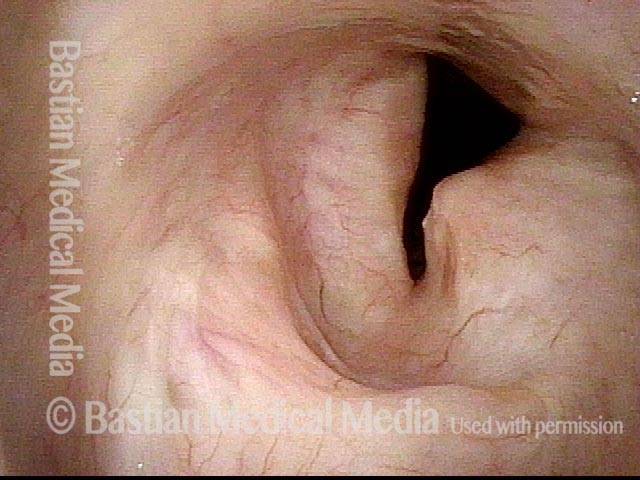
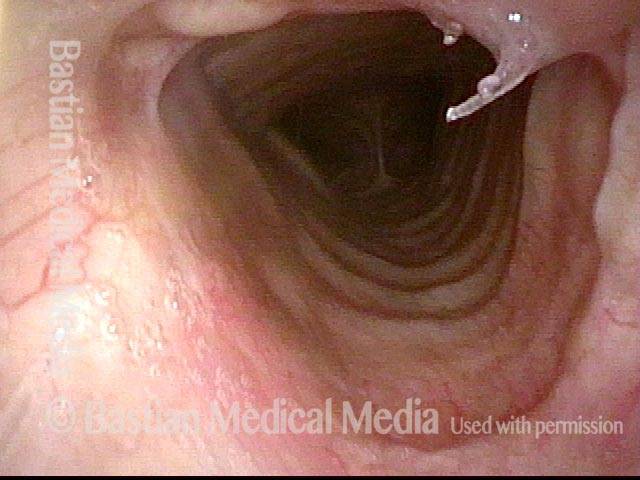
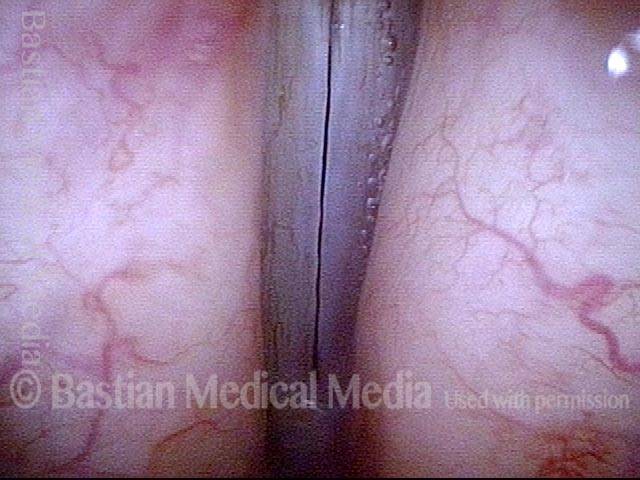
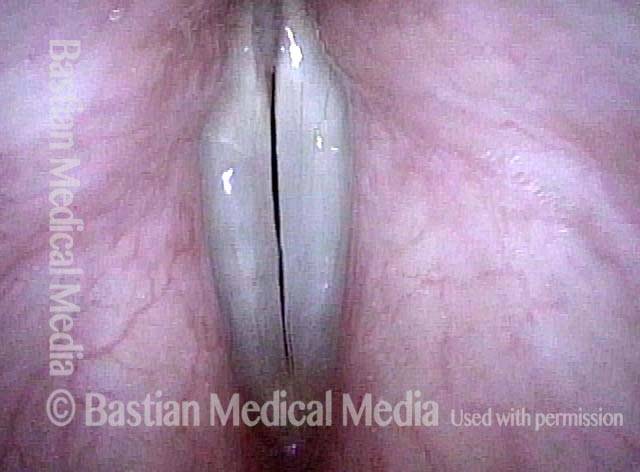
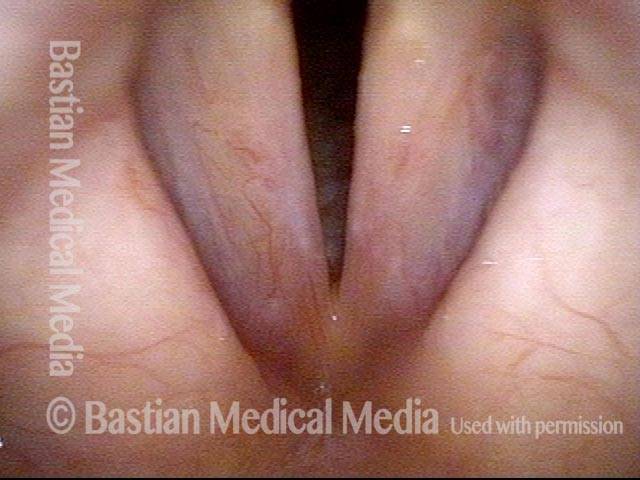
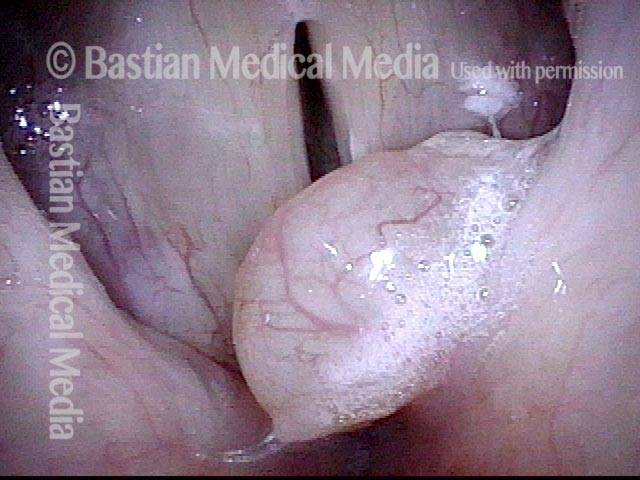
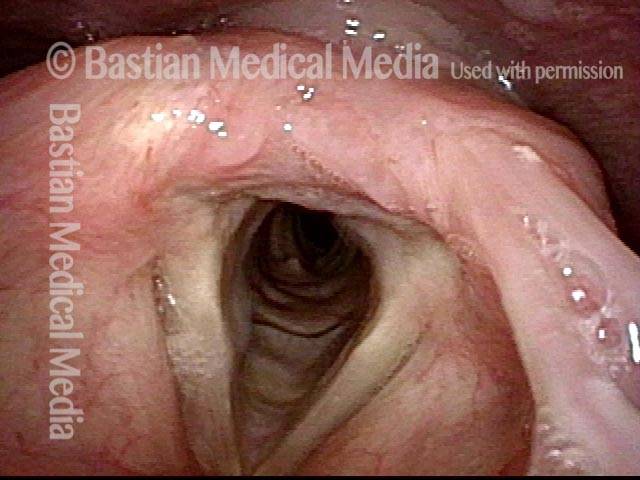
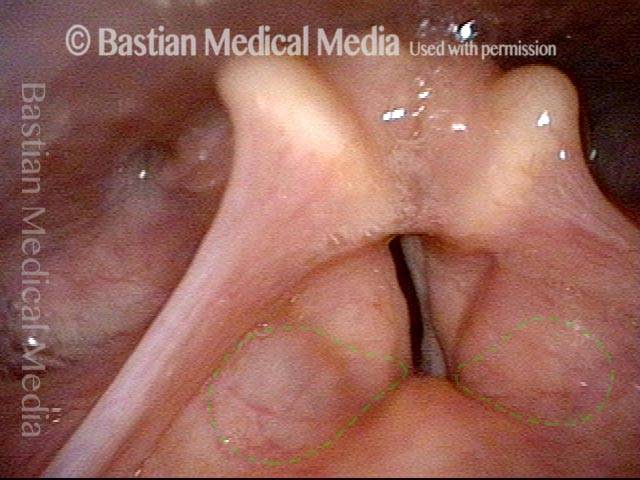
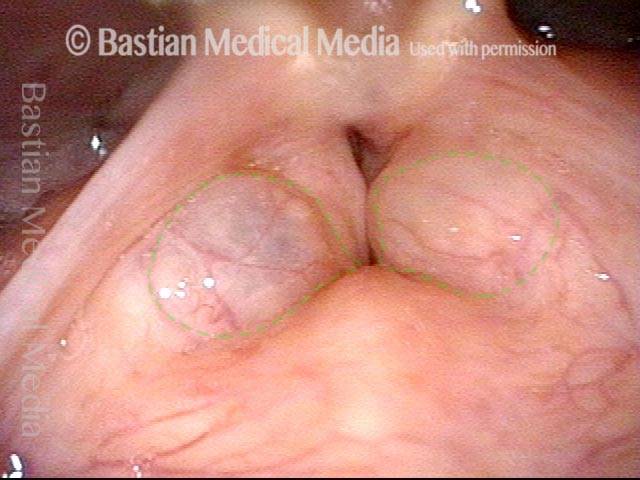
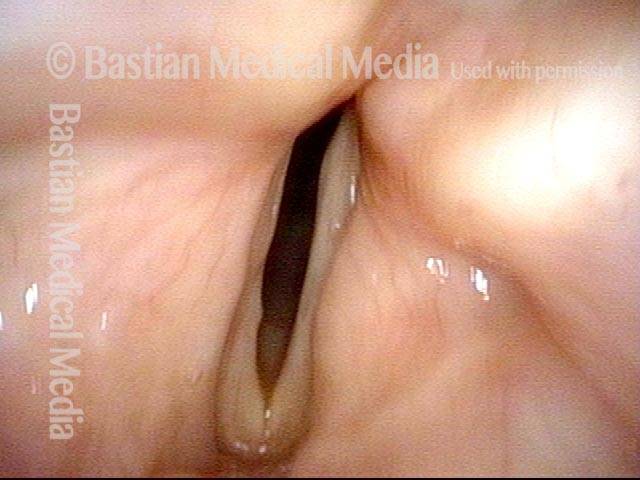
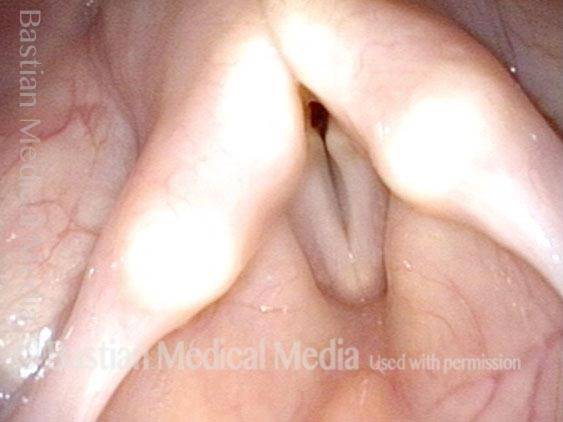
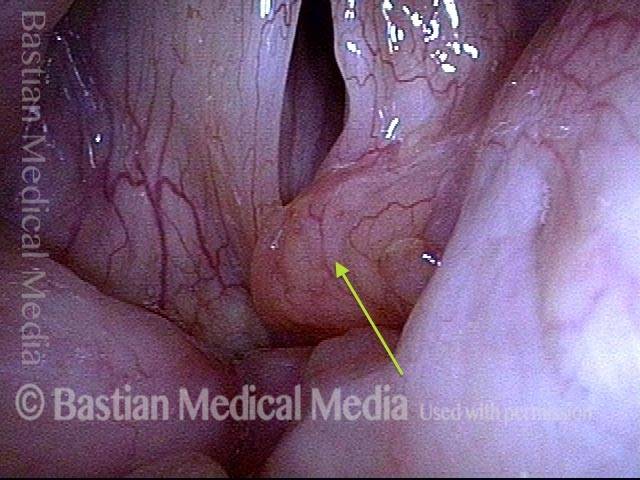
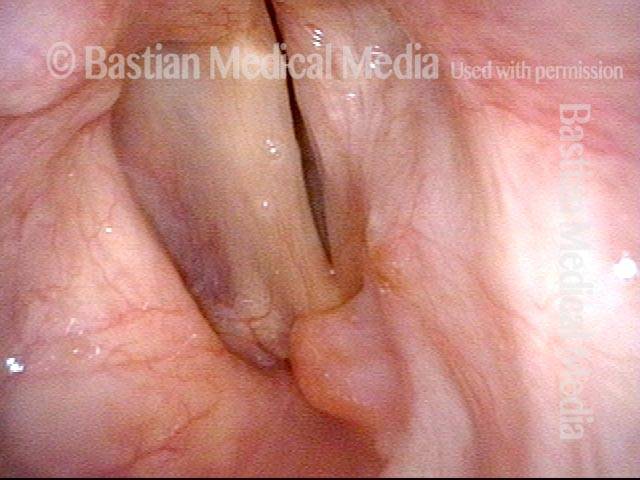
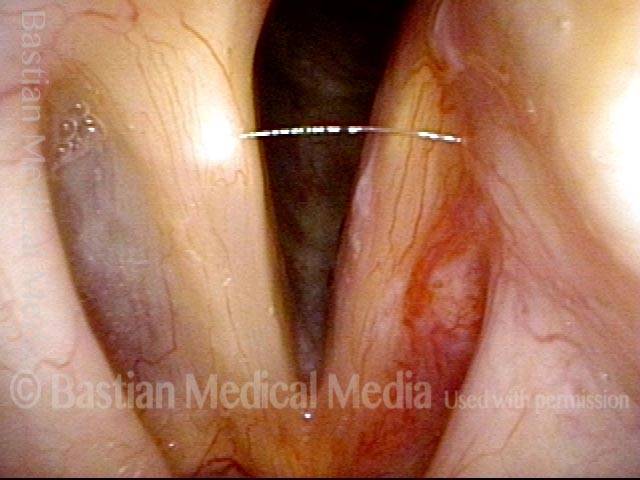
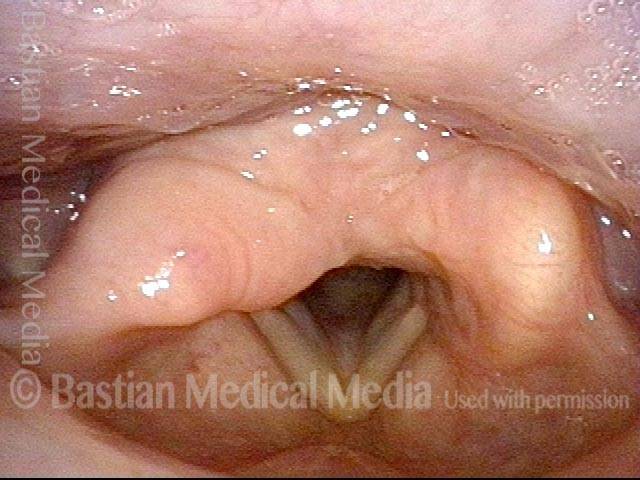
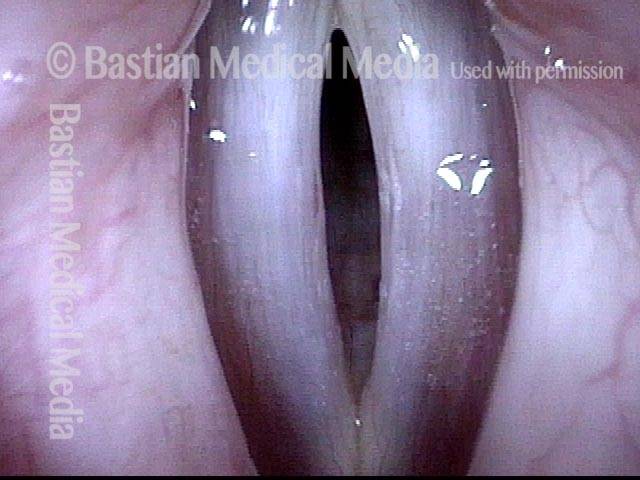
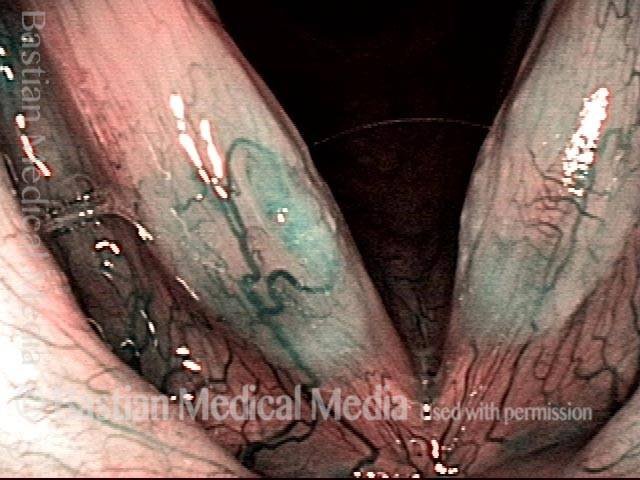
Closed phase (3 of 8)
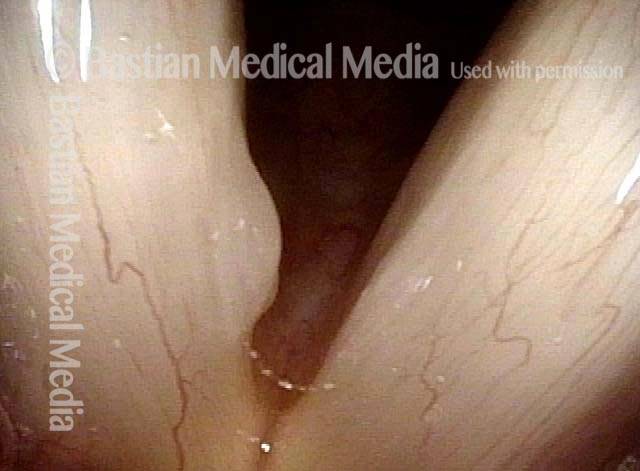
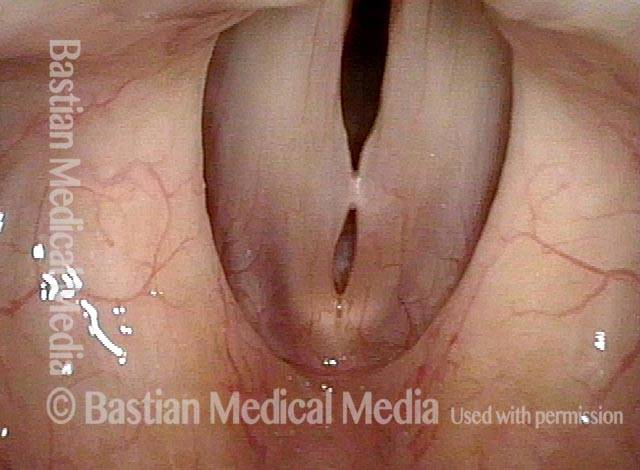
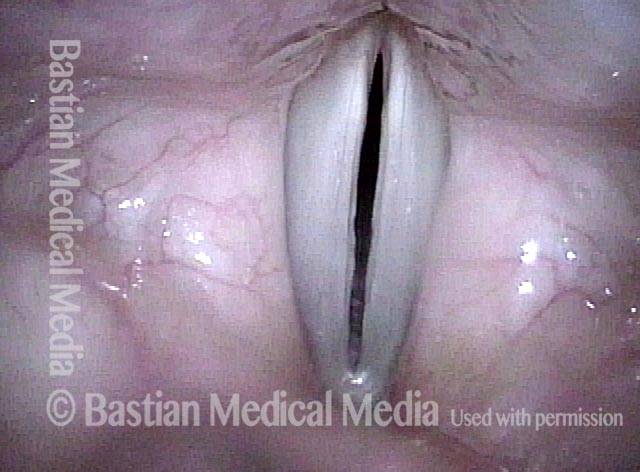
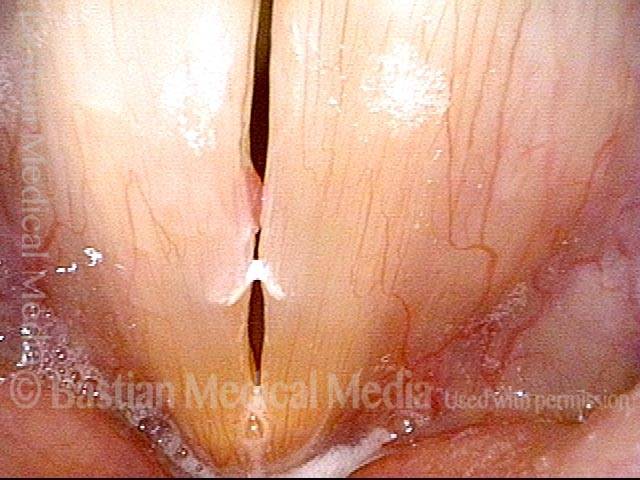
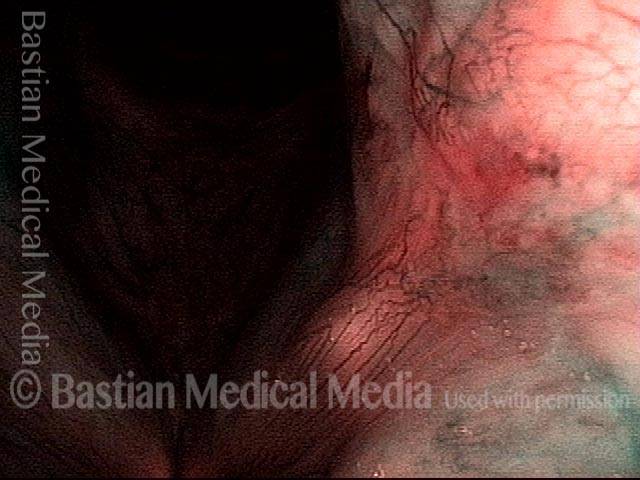
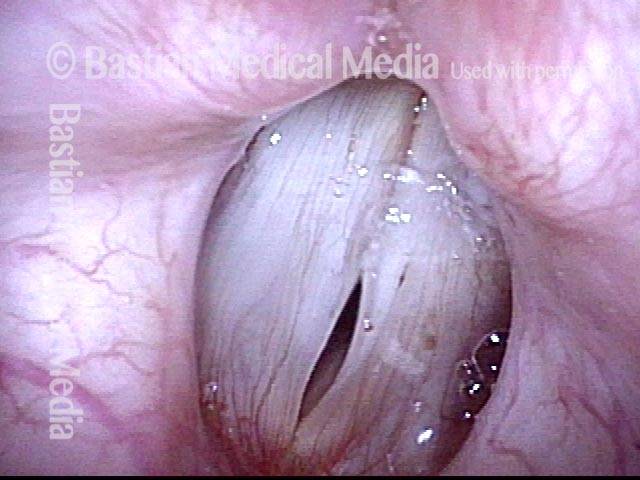
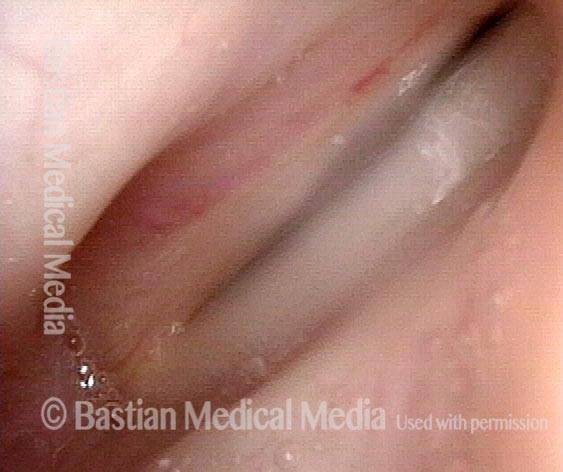
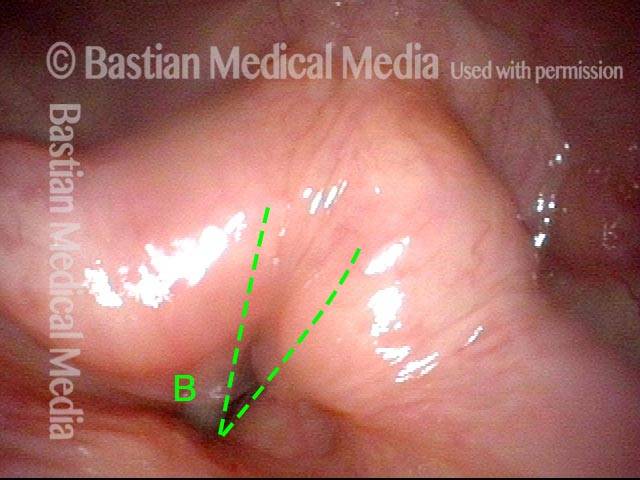
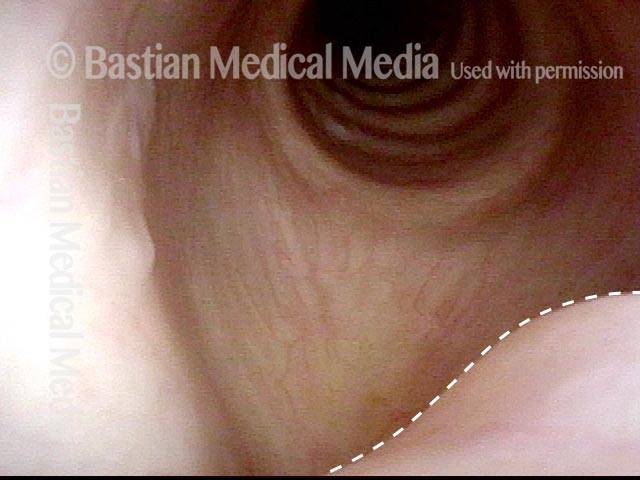
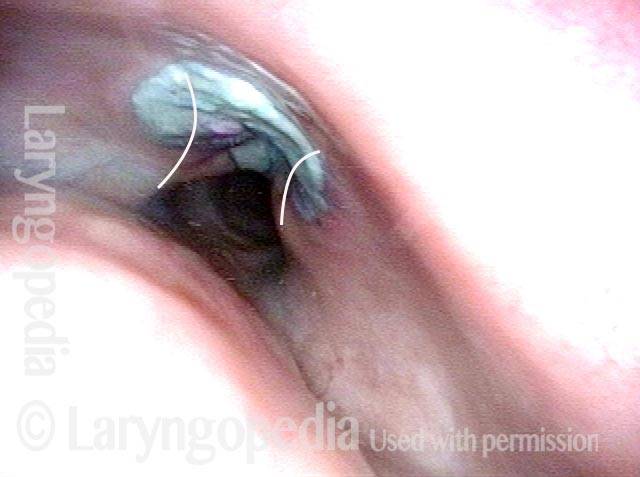
Closed phase of vibration (strobe light) at B flat 5 (932 Hz).
Closed phase (3 of 8)
Closed phase of vibration (strobe light) at B flat 5 (932 Hz).
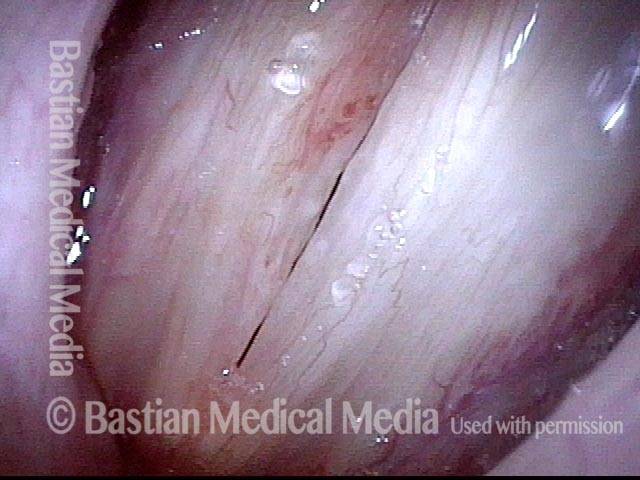
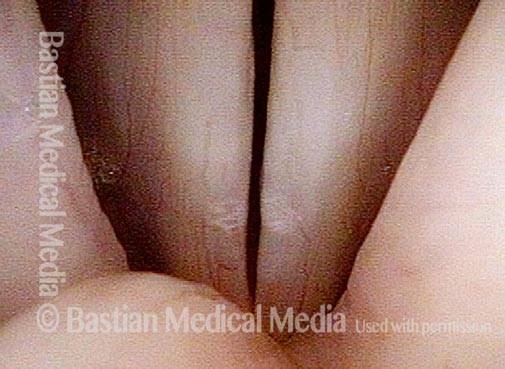
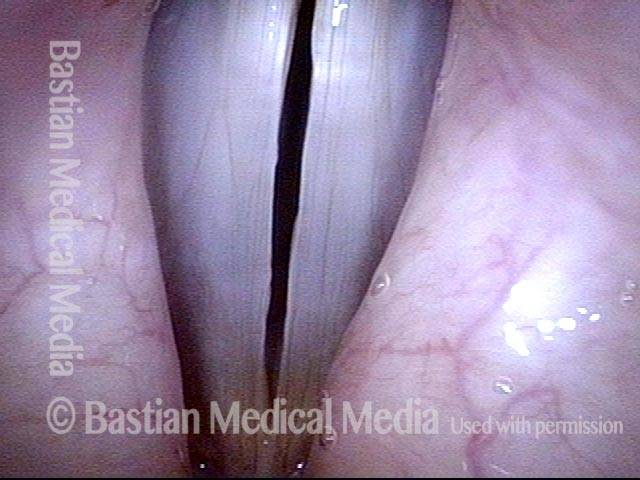
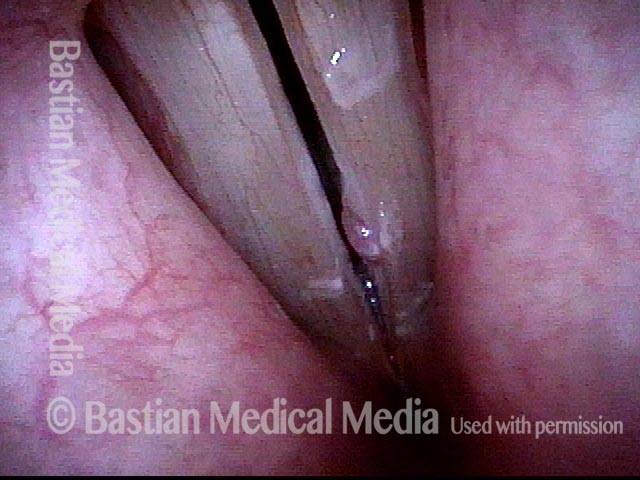
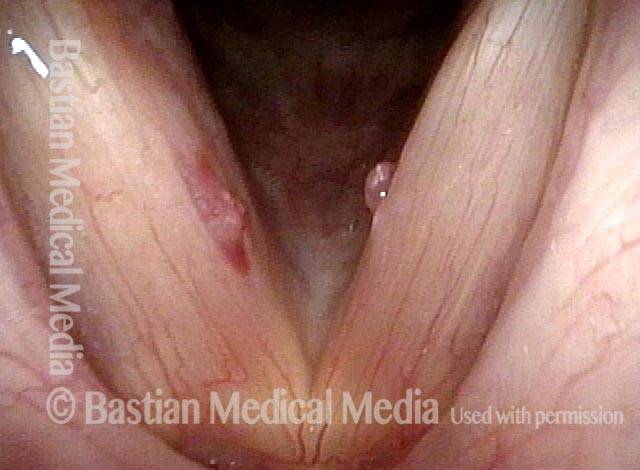
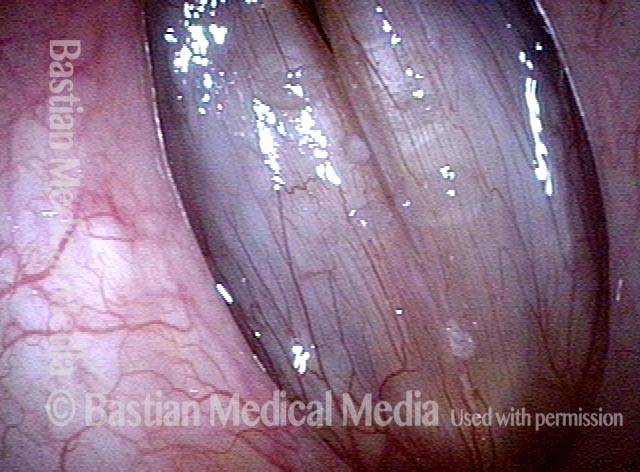
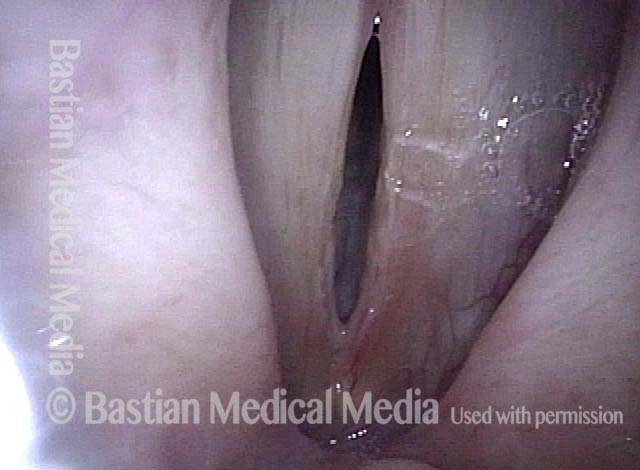
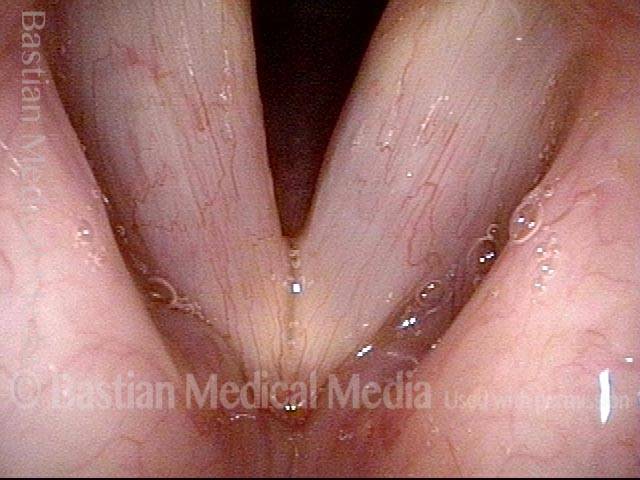
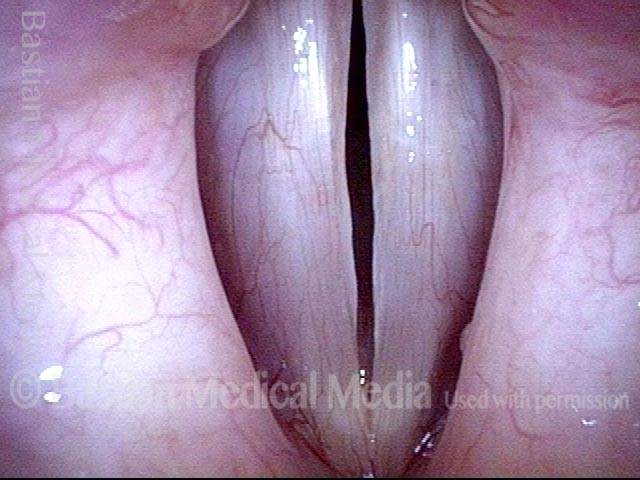
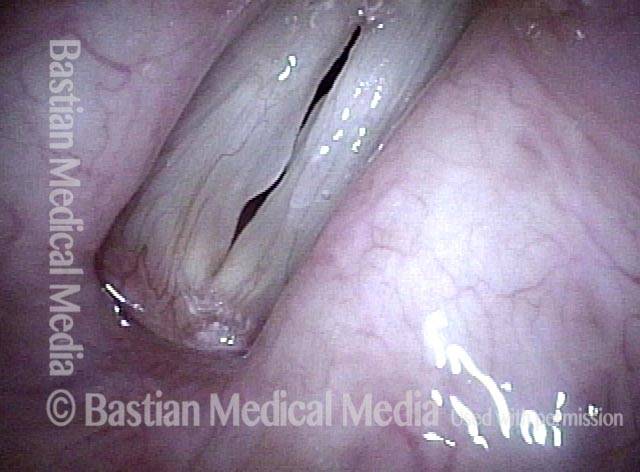
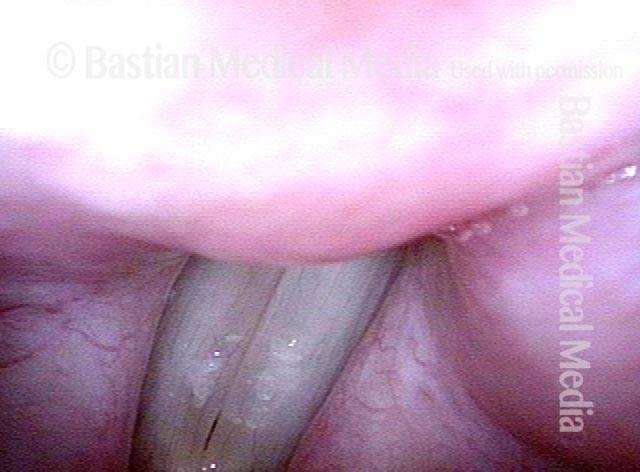
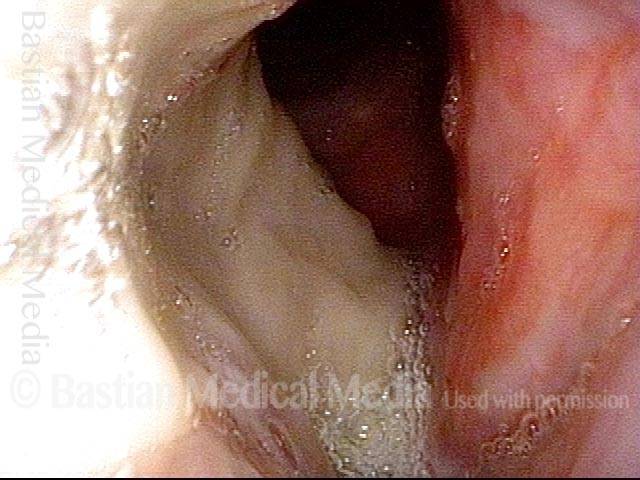
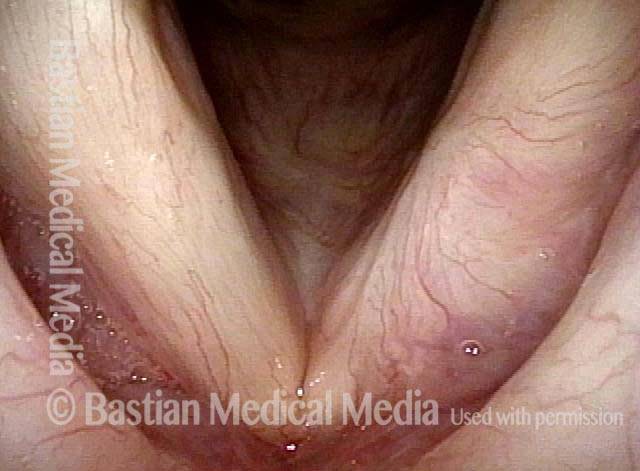
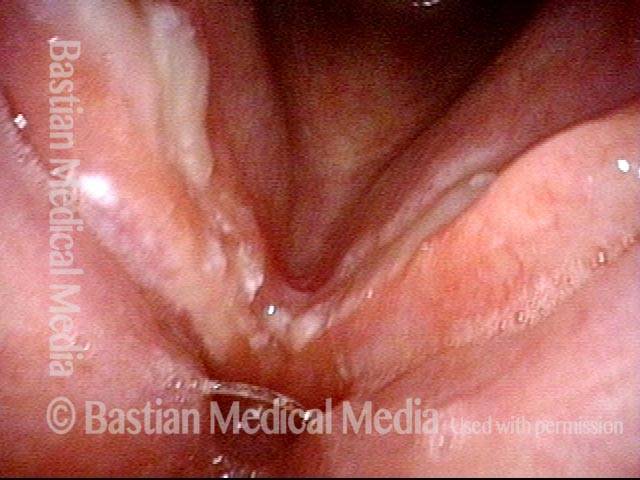
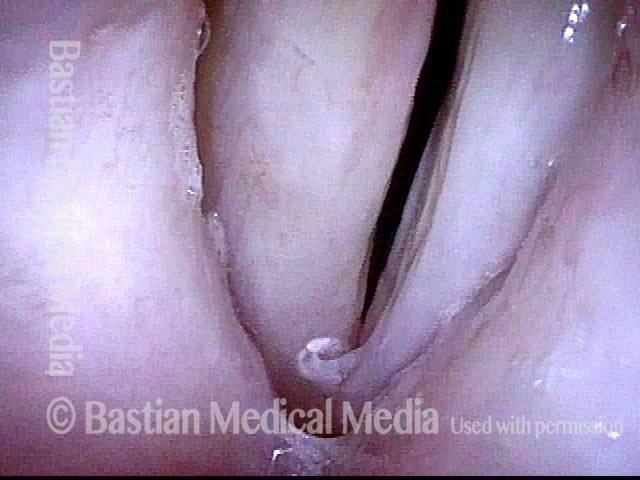
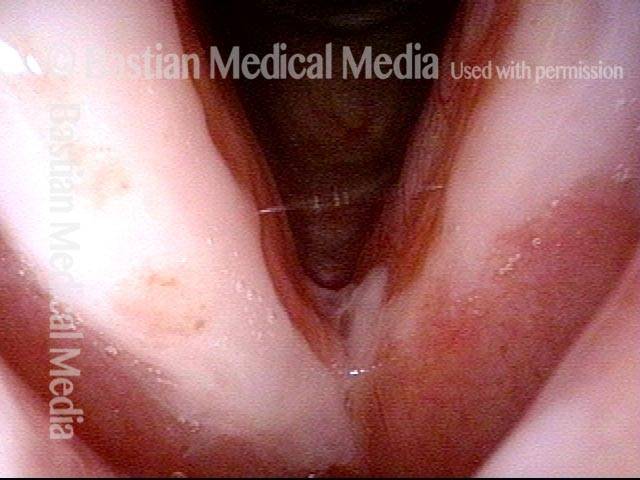
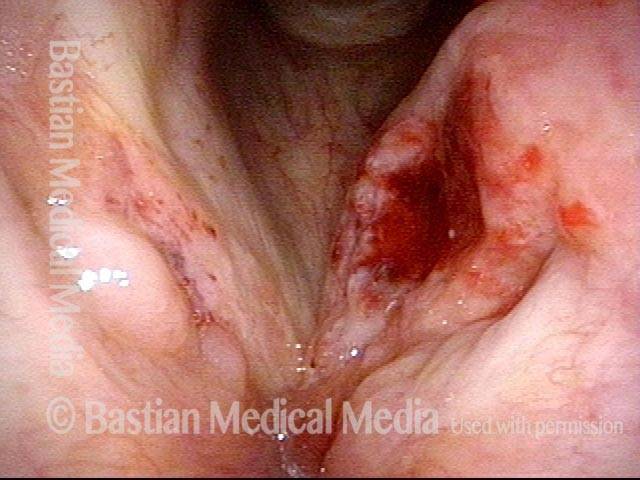
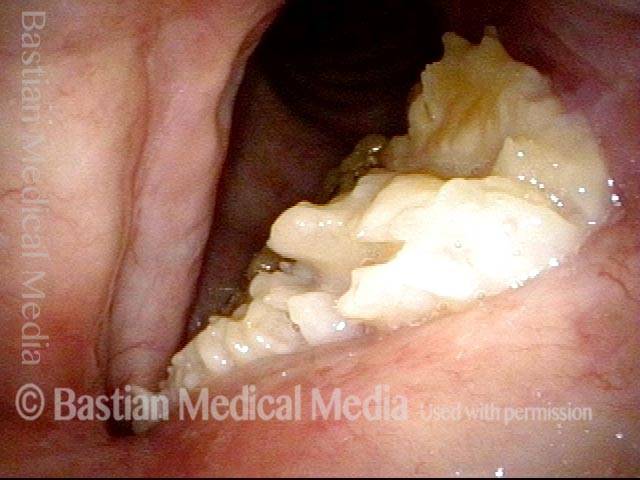
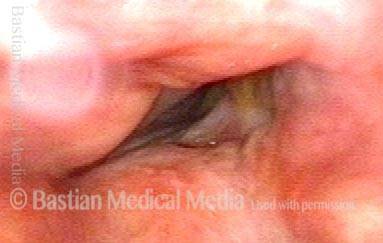
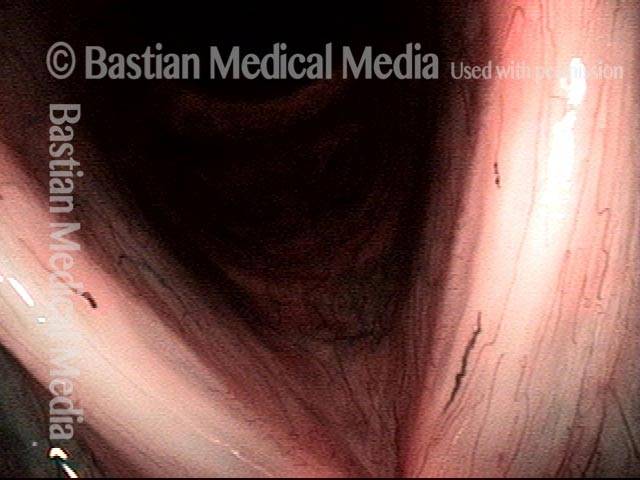
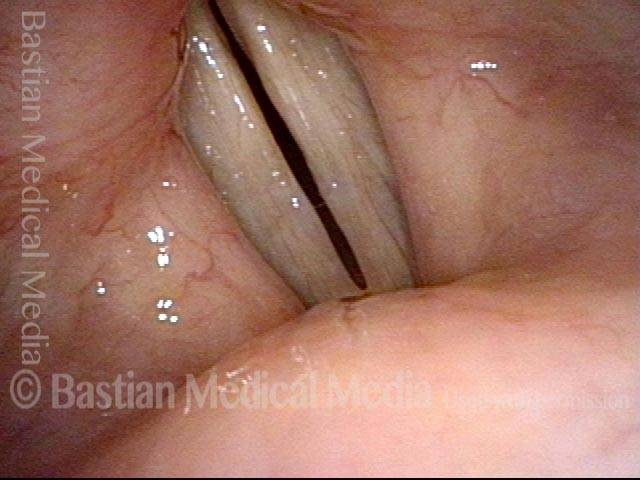
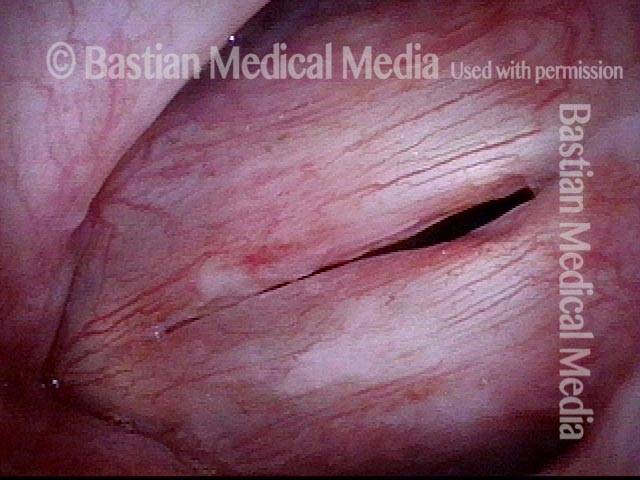
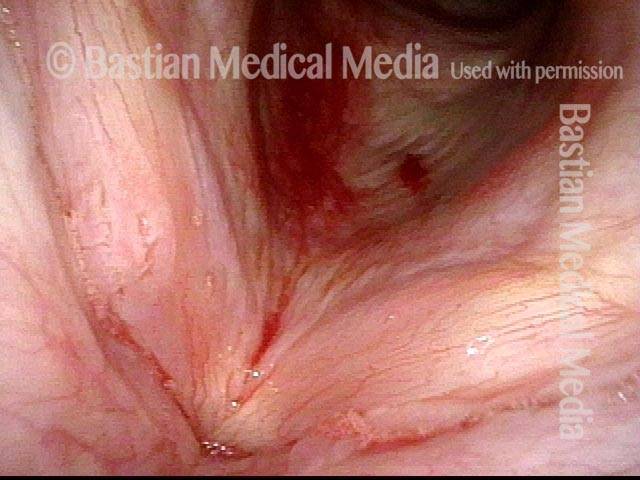
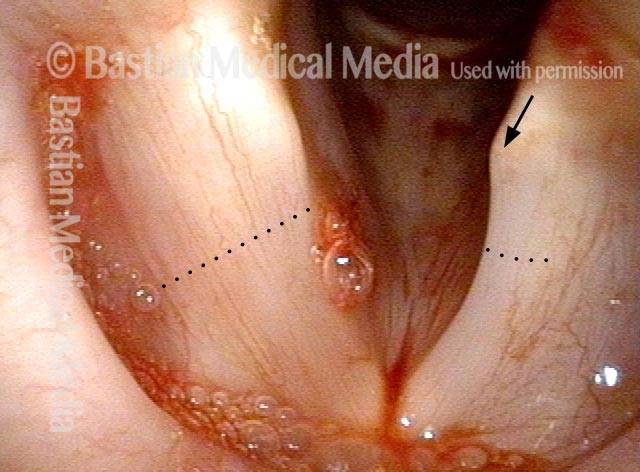
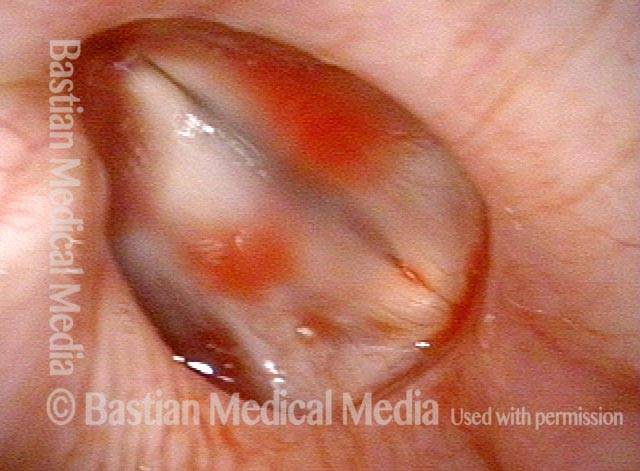
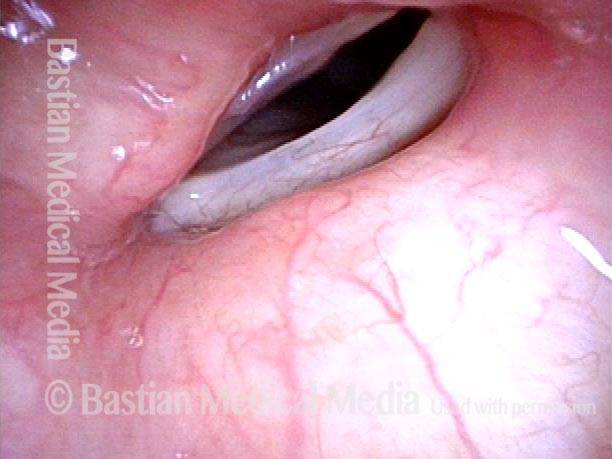
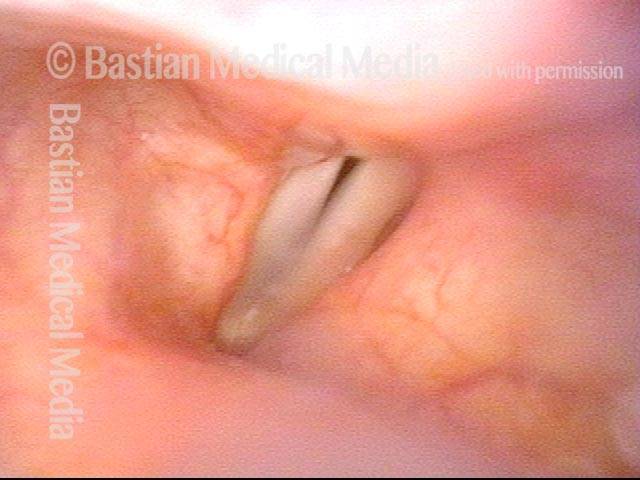
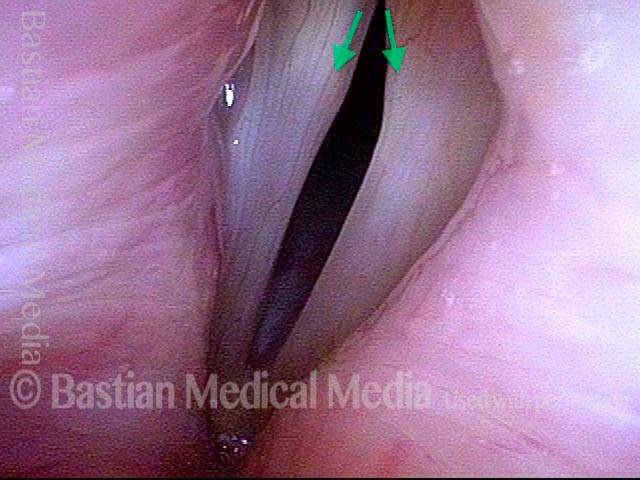
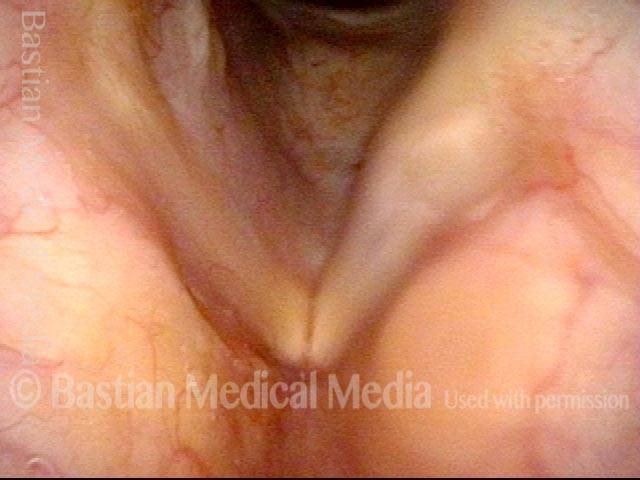
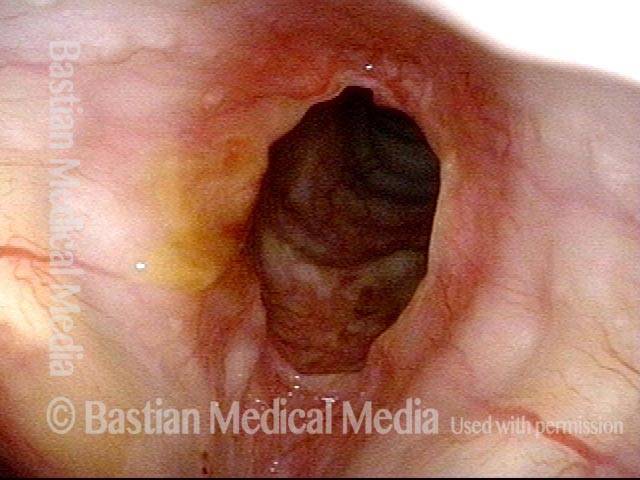
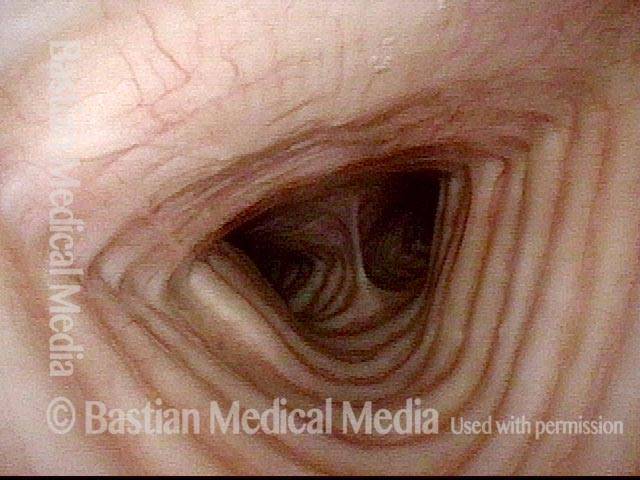
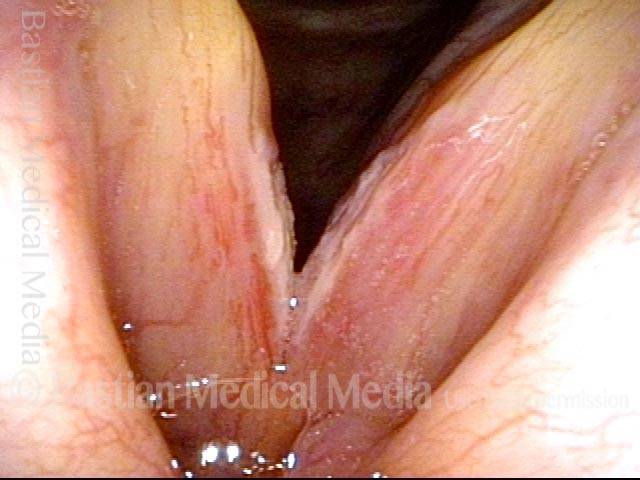
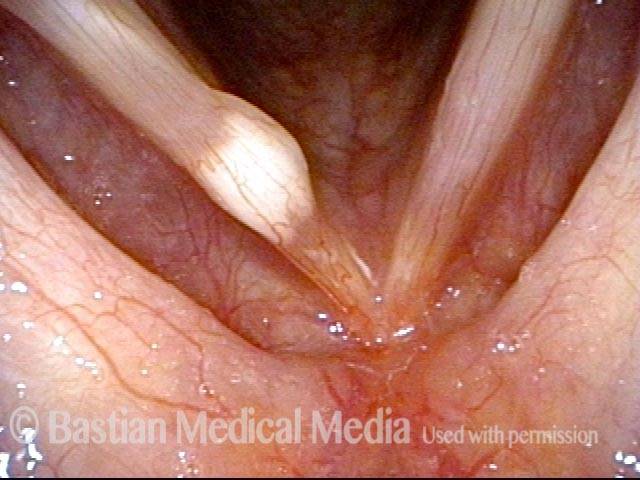
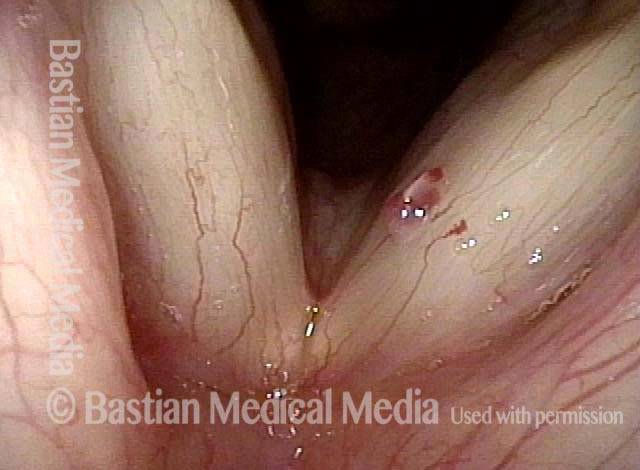
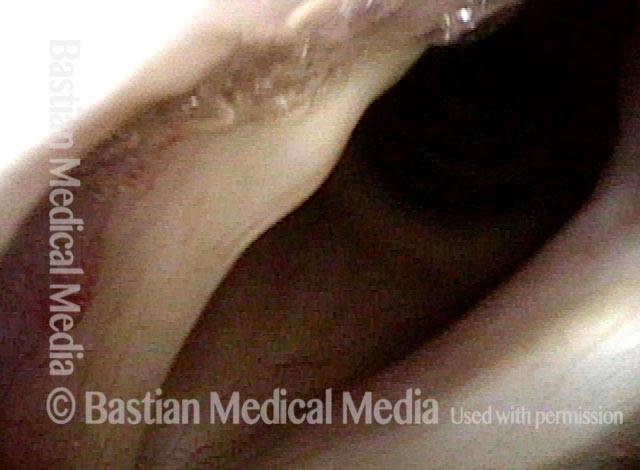
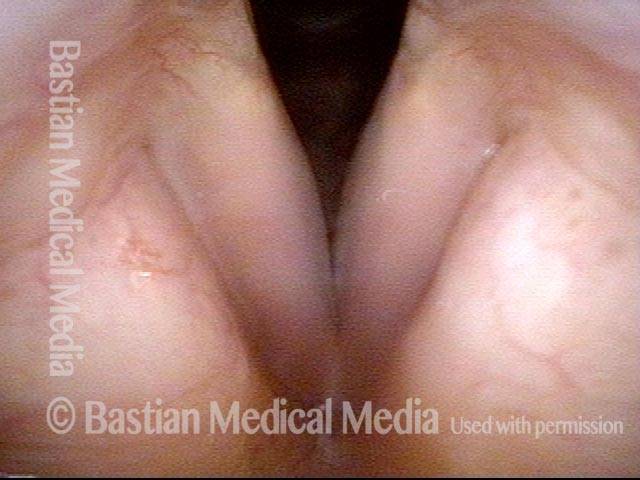
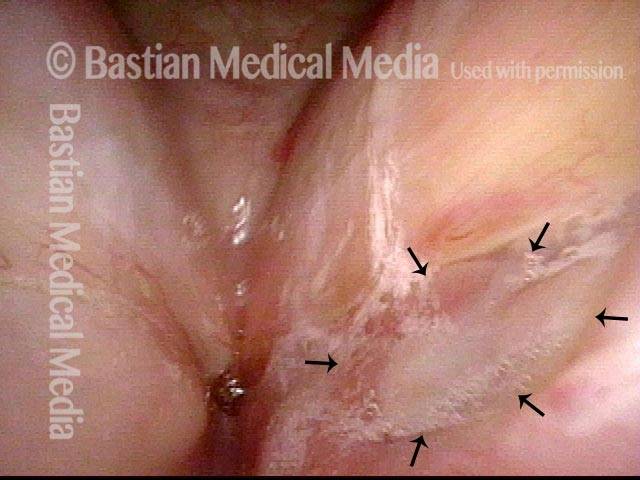
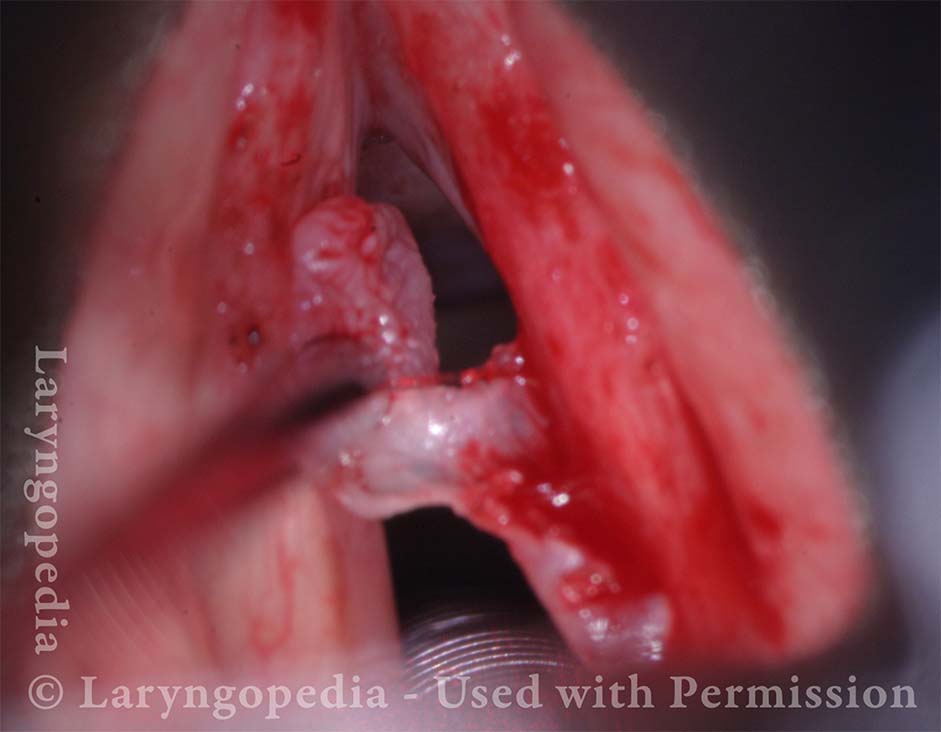
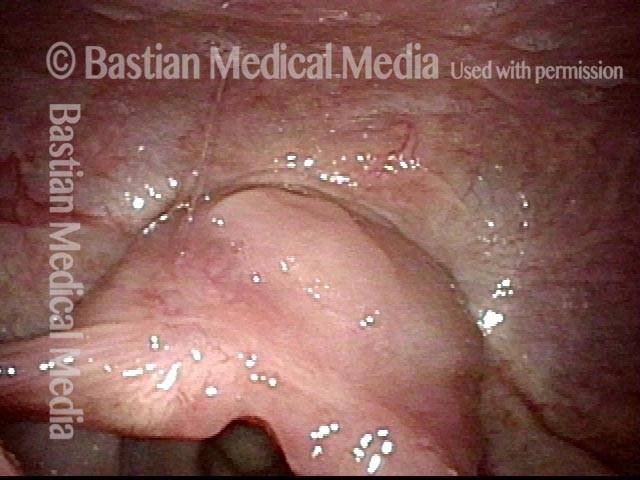
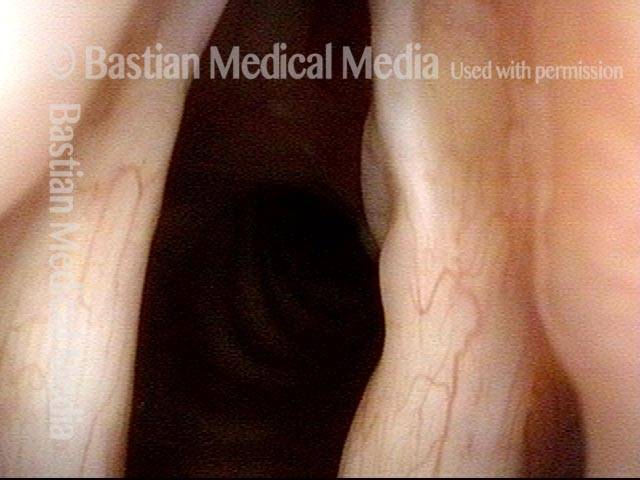
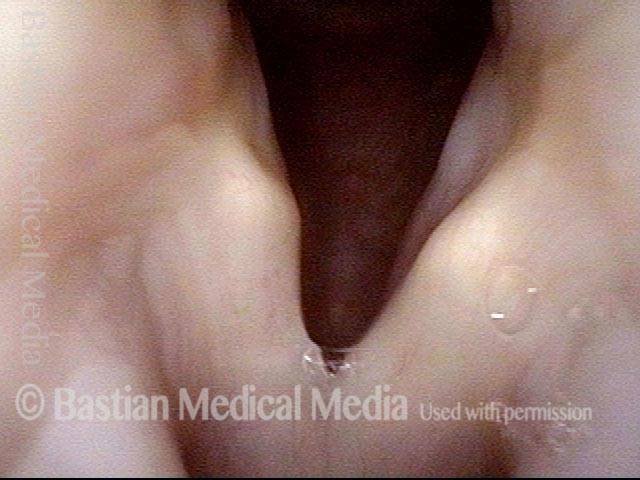
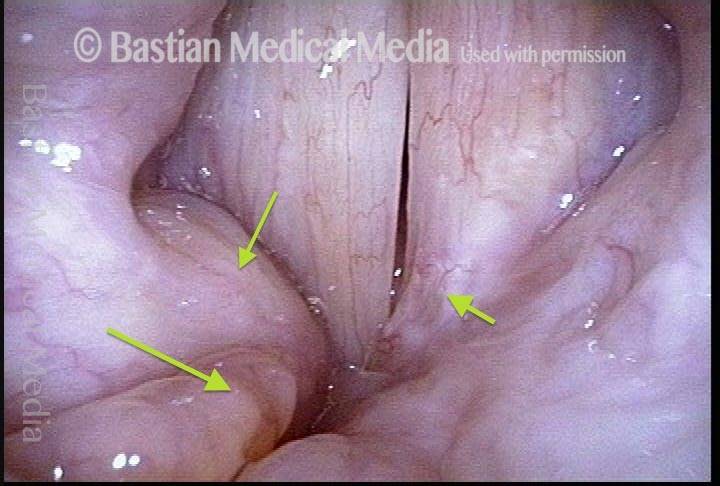
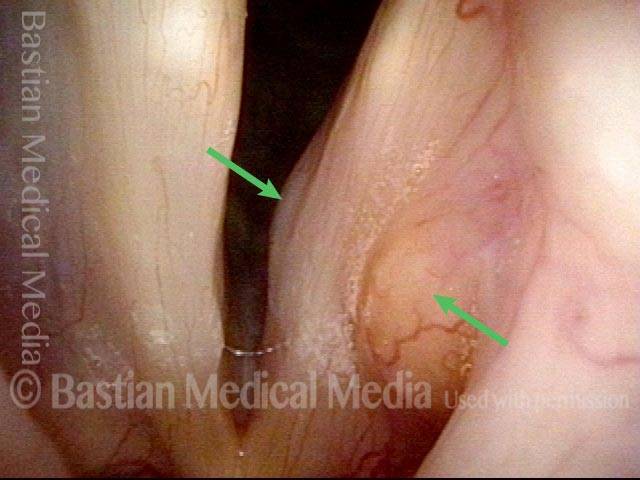
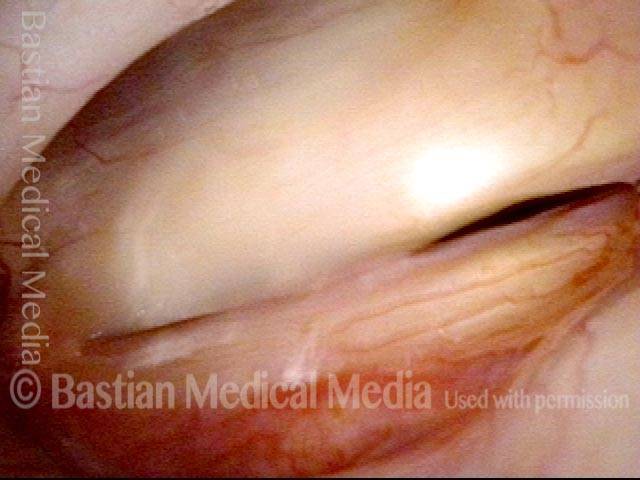
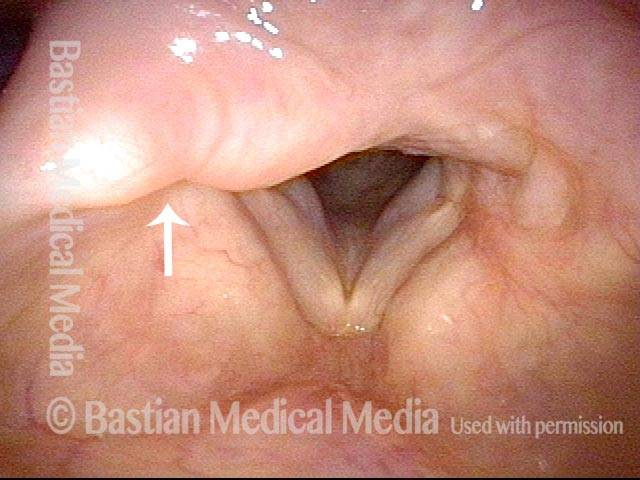
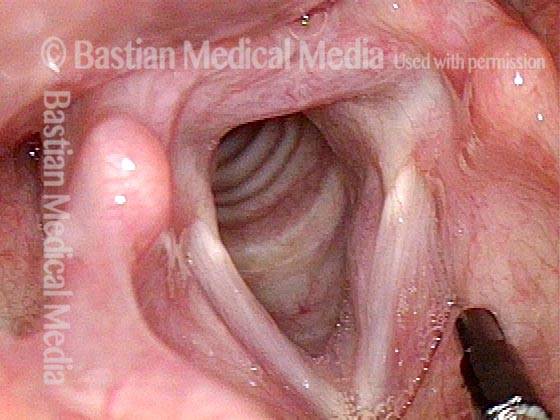
24 hours post surgery (5 of 8)
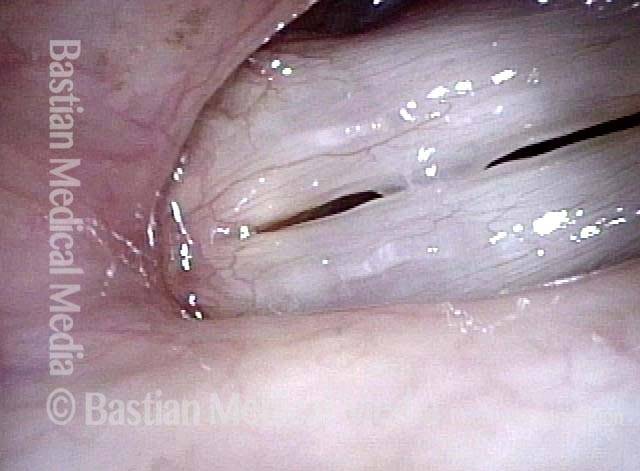
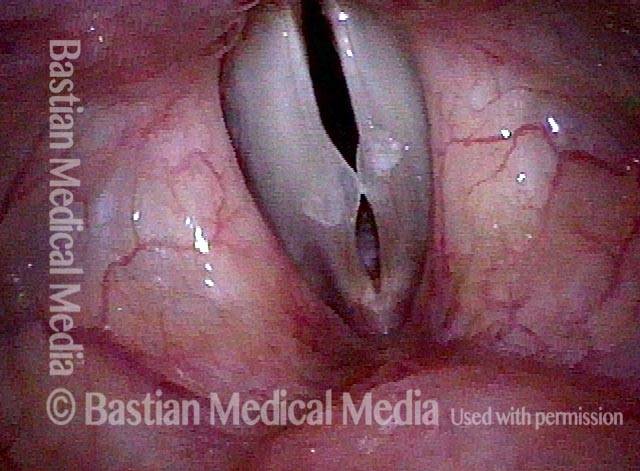
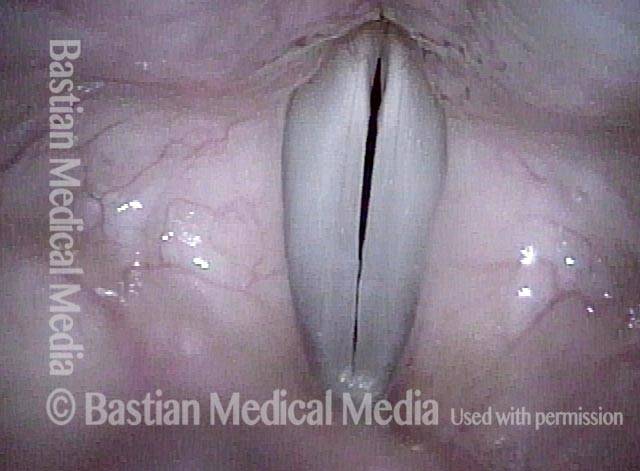
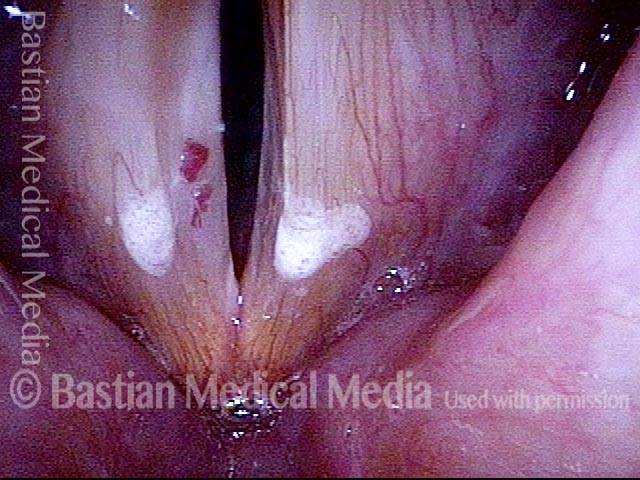
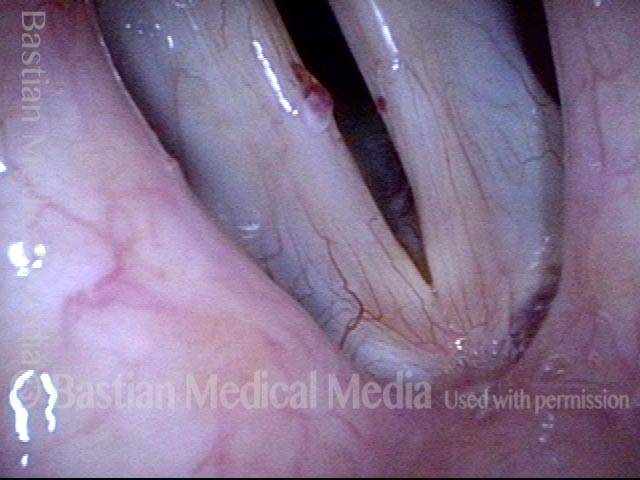
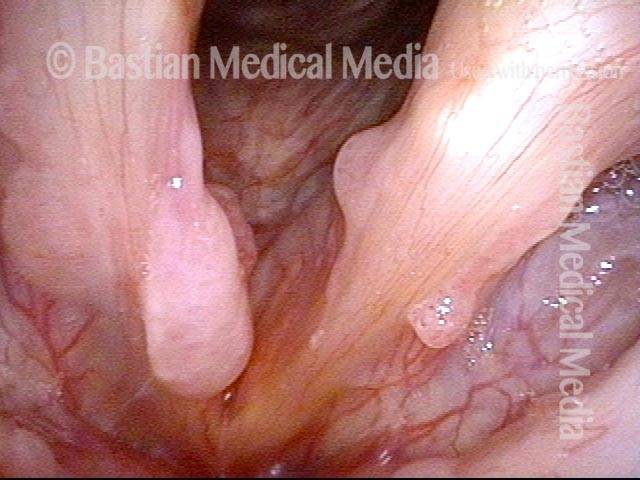
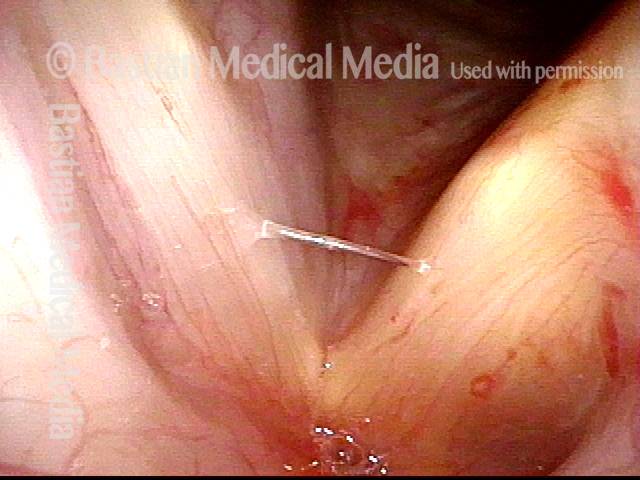
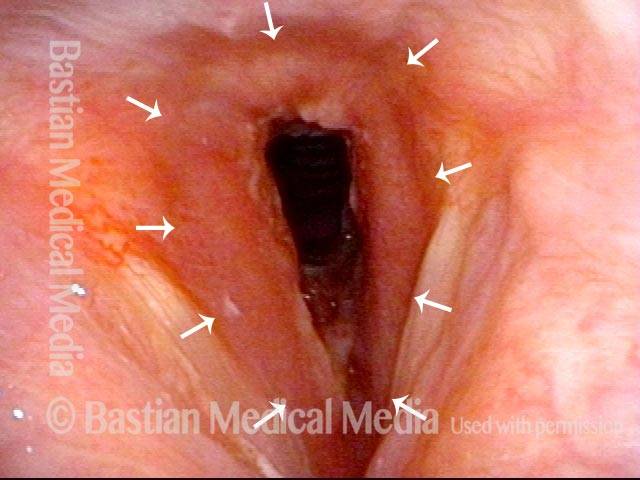
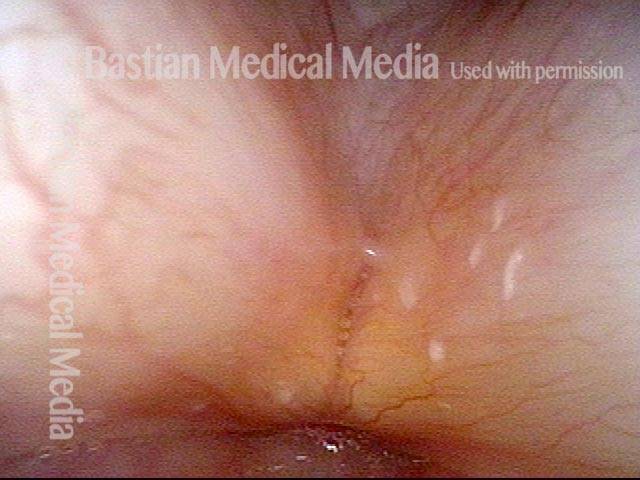
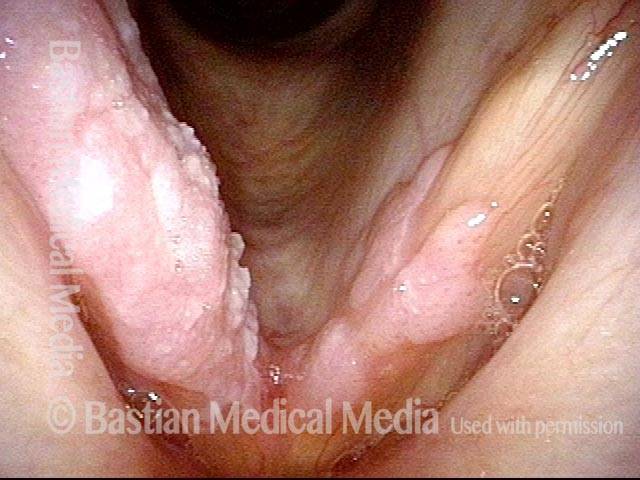
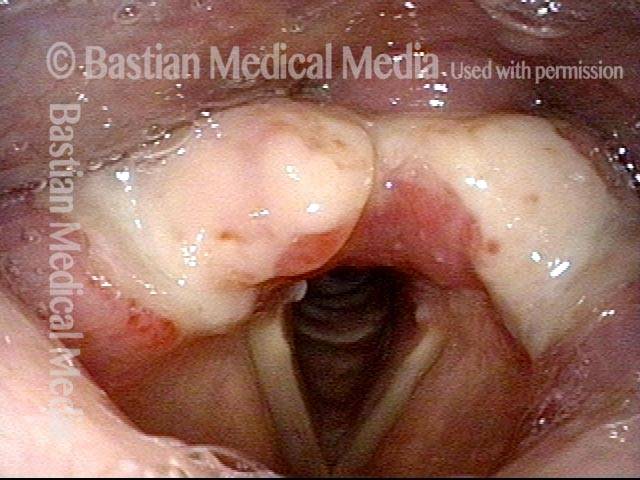
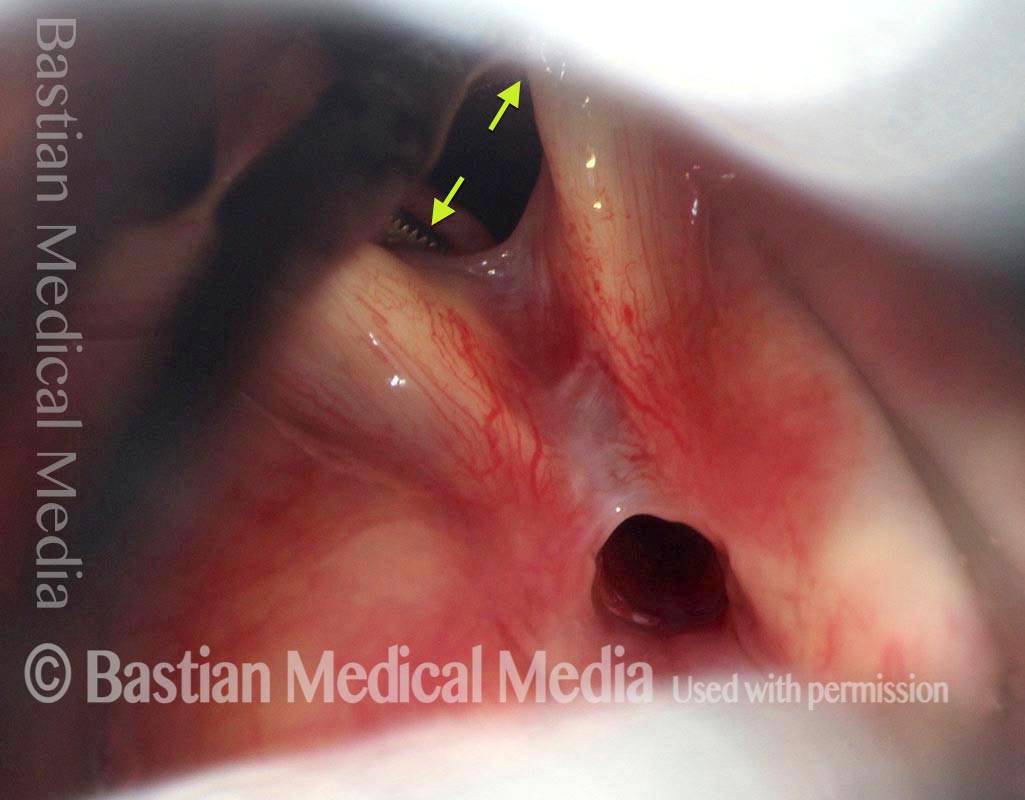
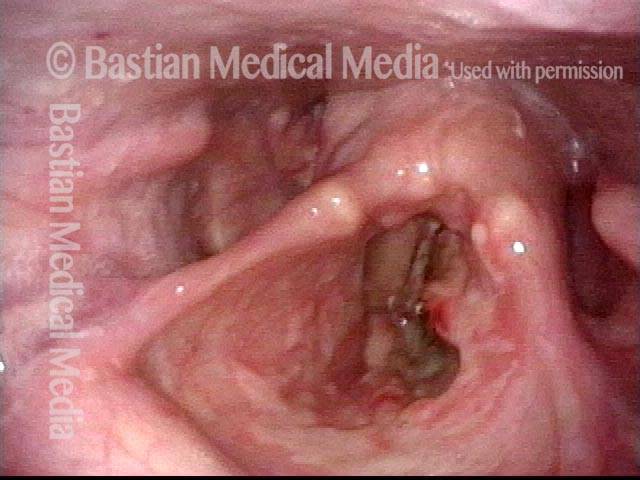
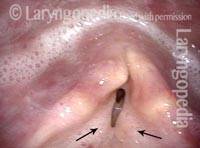
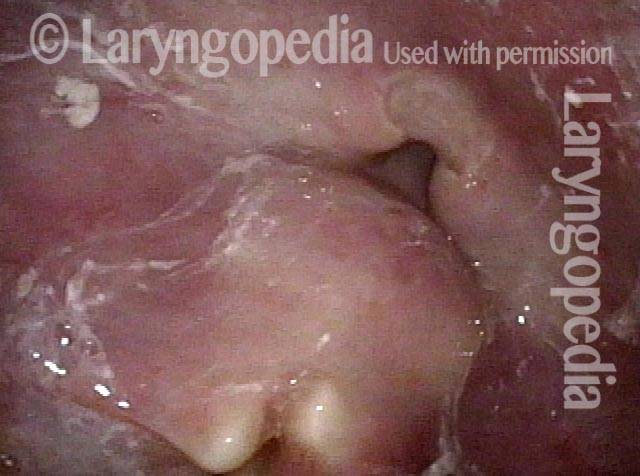
The next day, only a few hours after surgical removal. Note slight bruising from the endotracheal tube (long arrows), and small dots where a laser impact was used to interrupt flow in a prominent capillary (short arrows).
24 hours post surgery (5 of 8)
The next day, only a few hours after surgical removal. Note slight bruising from the endotracheal tube (long arrows), and small dots where a laser impact was used to interrupt flow in a prominent capillary (short arrows).
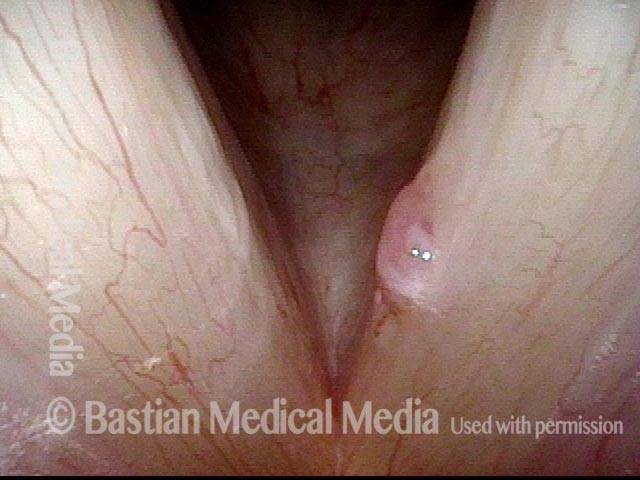
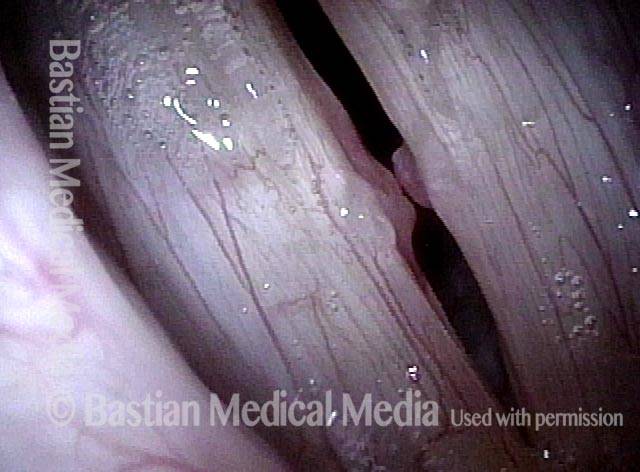
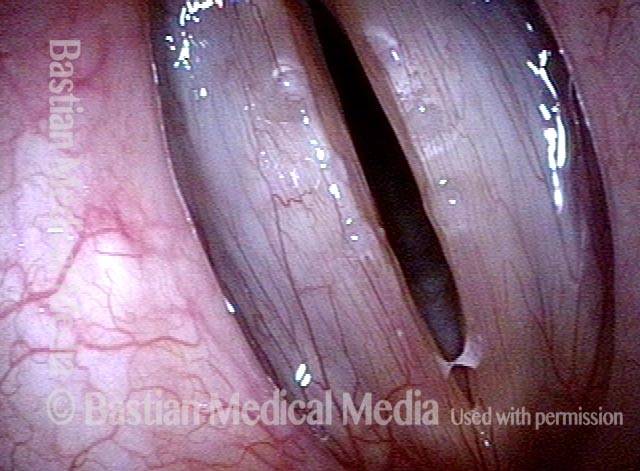
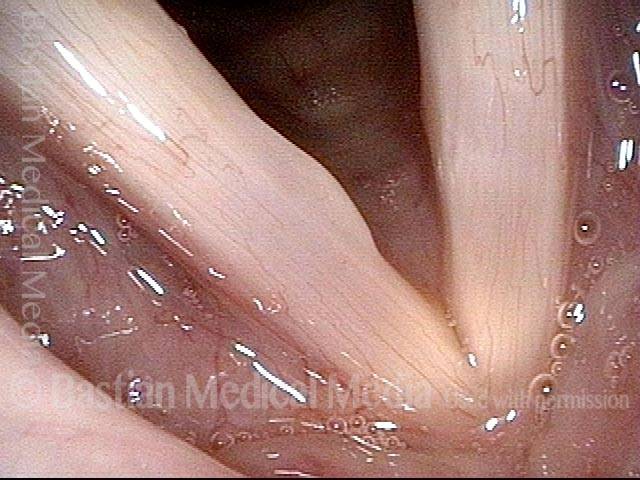
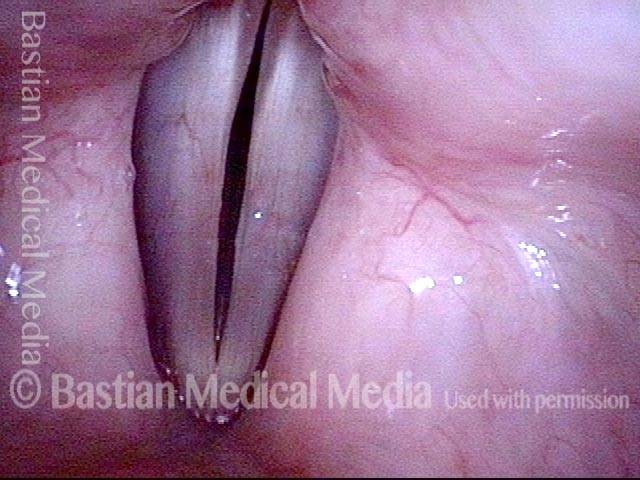
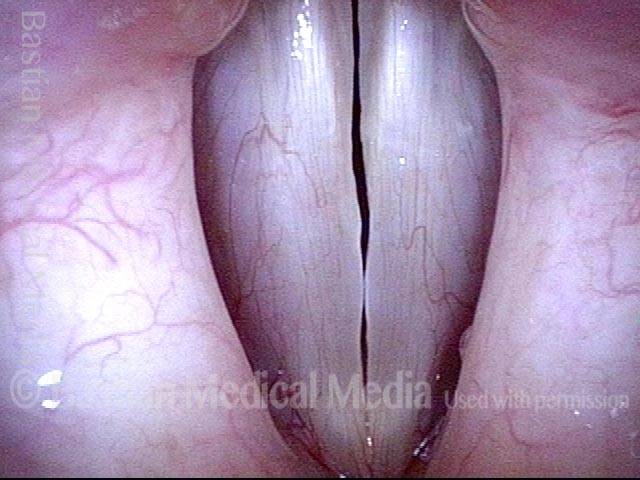
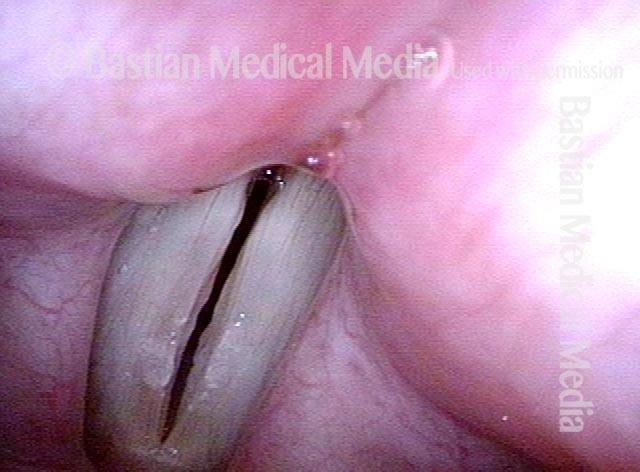
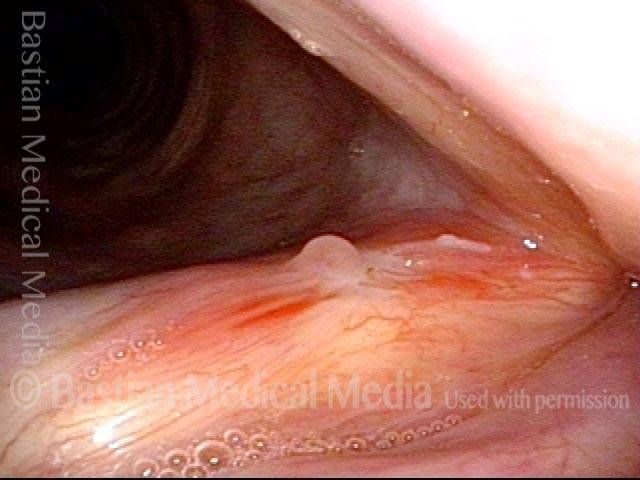
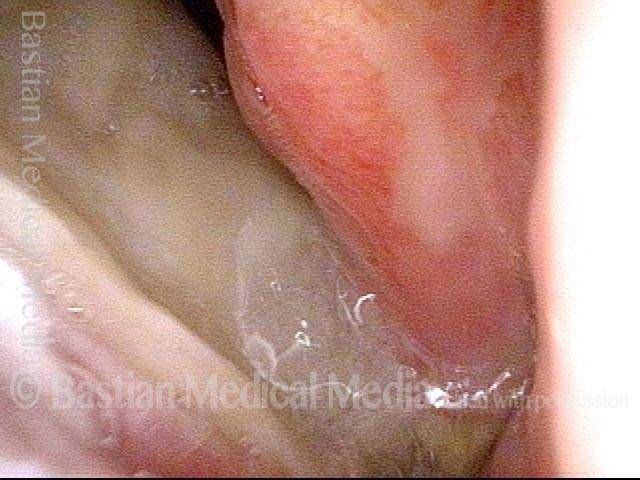
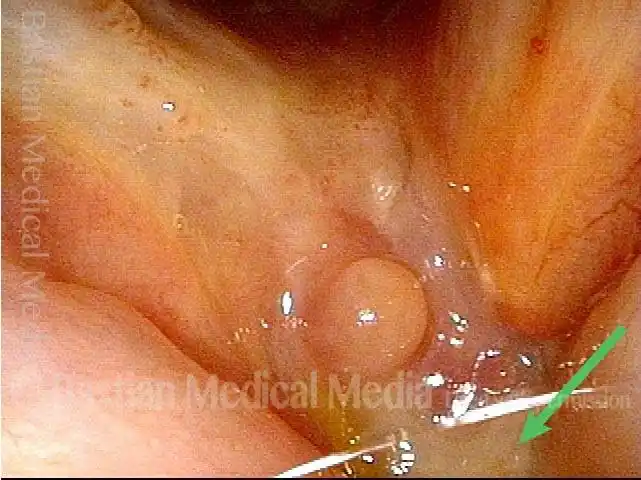
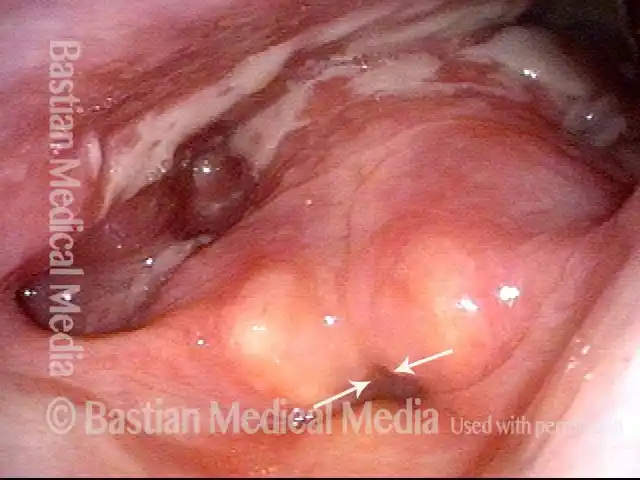
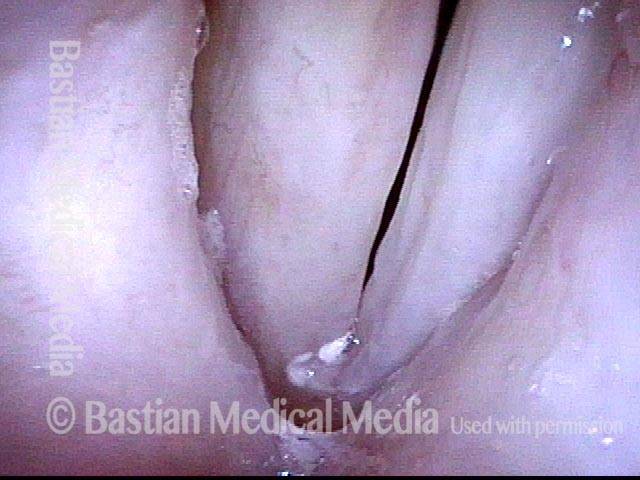
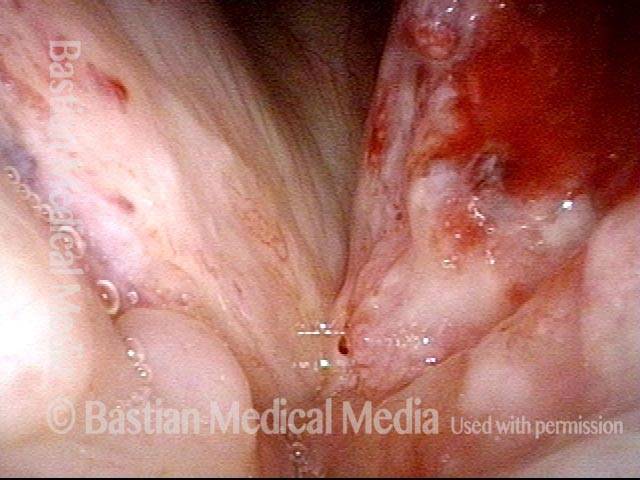
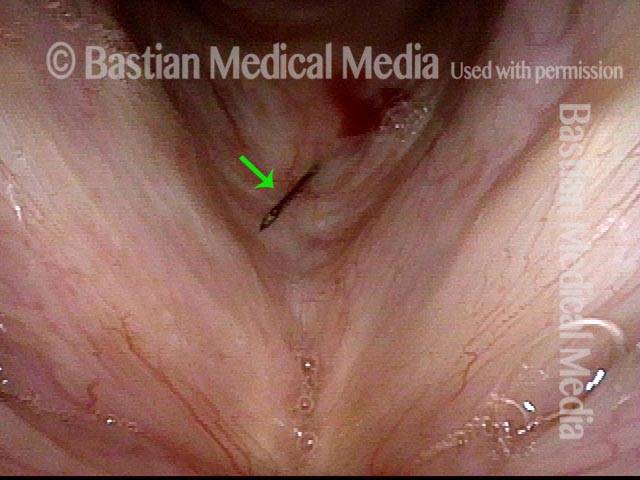
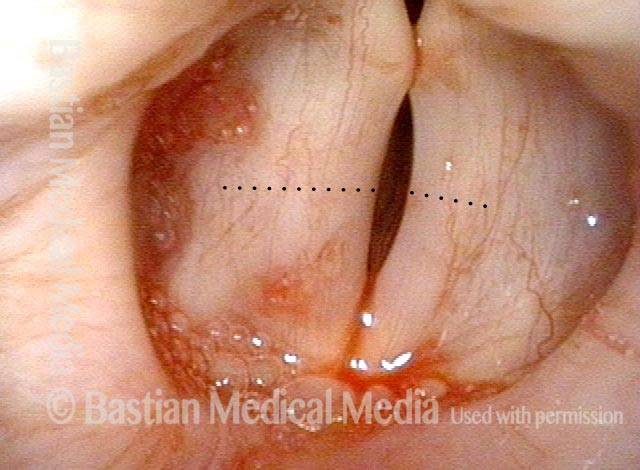
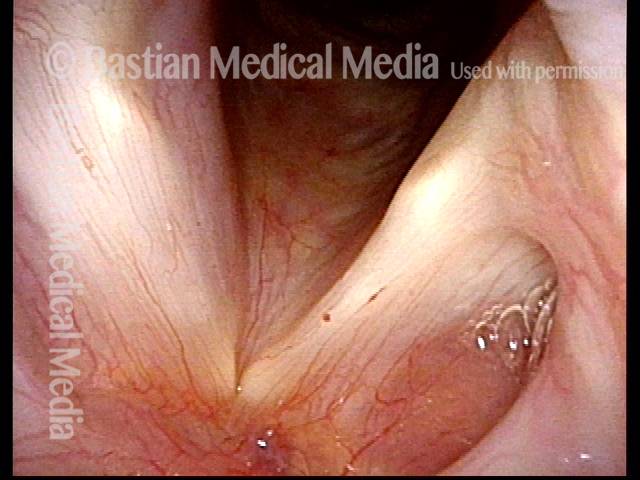
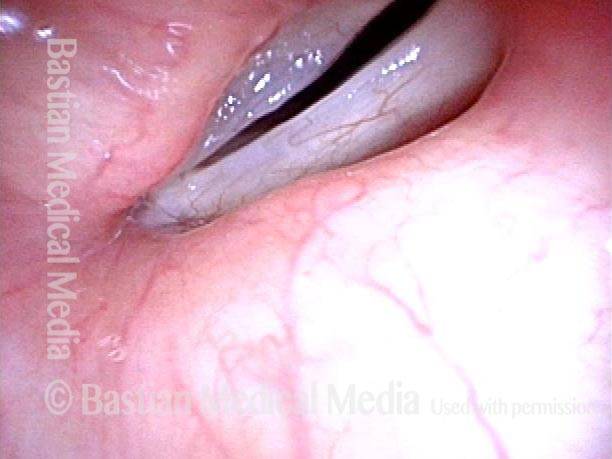
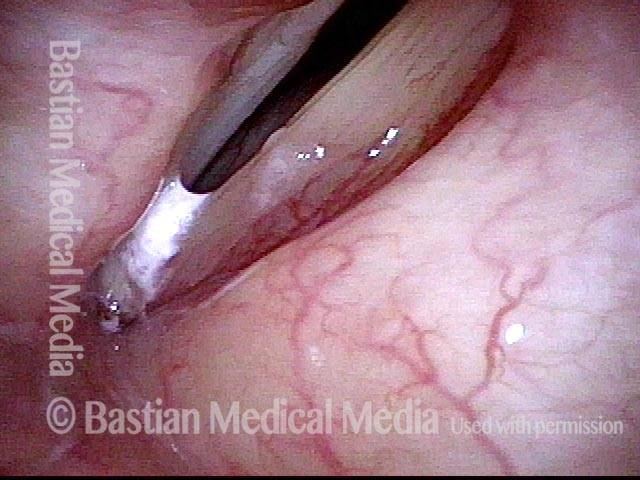
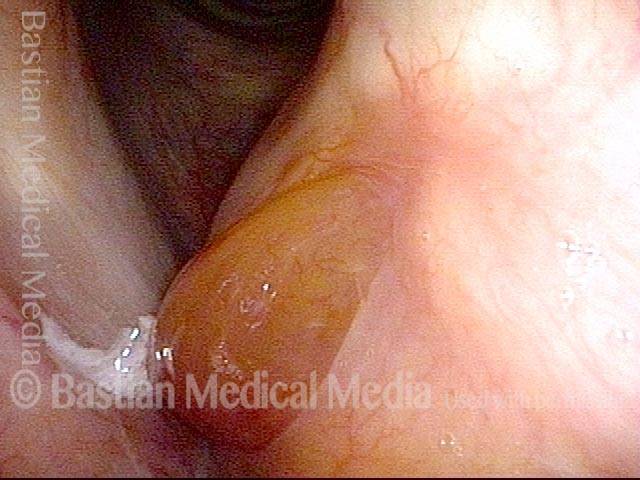
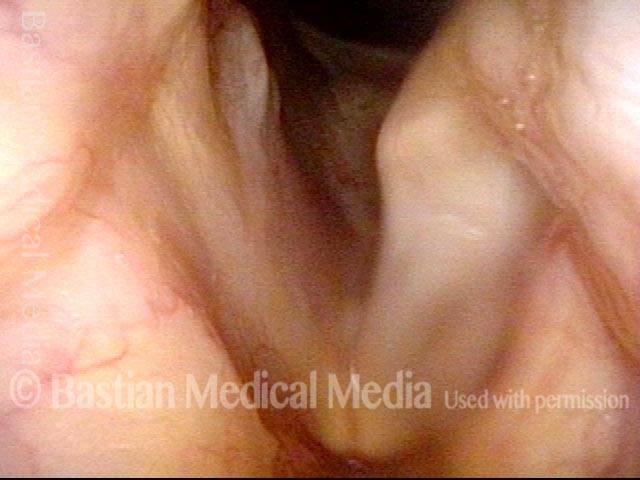
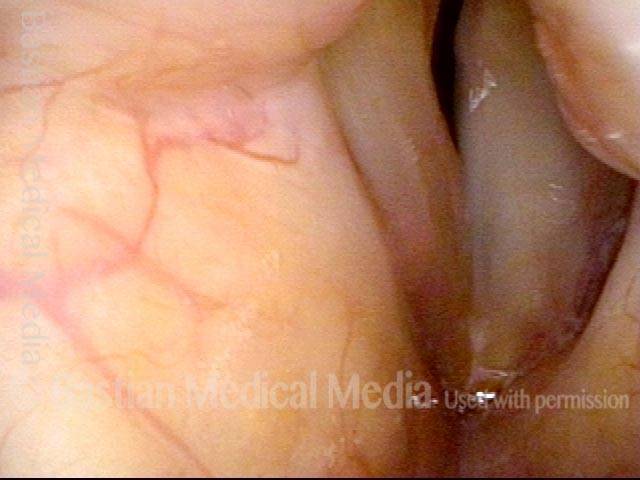
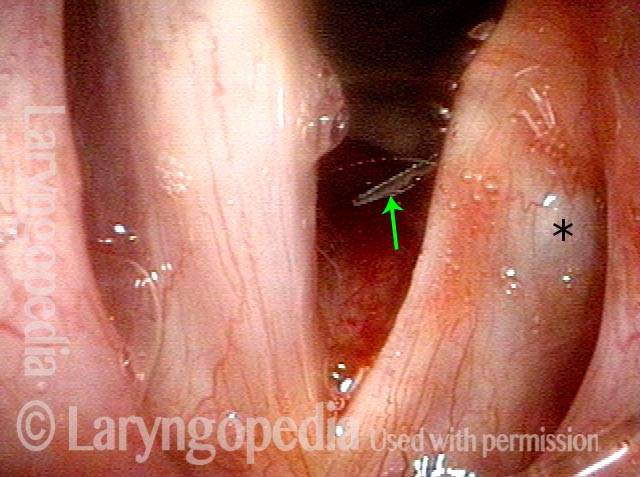
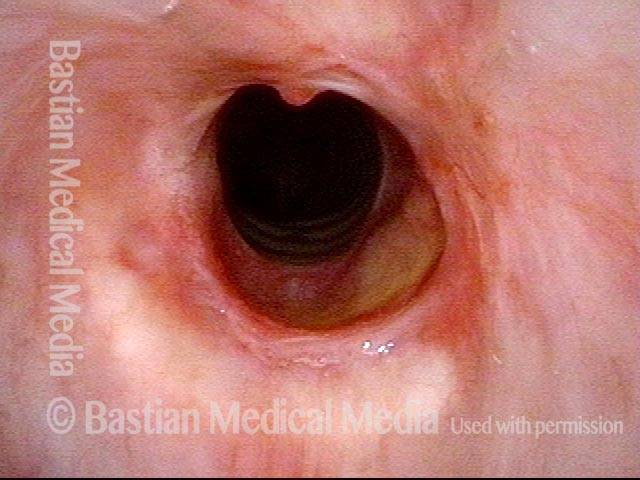
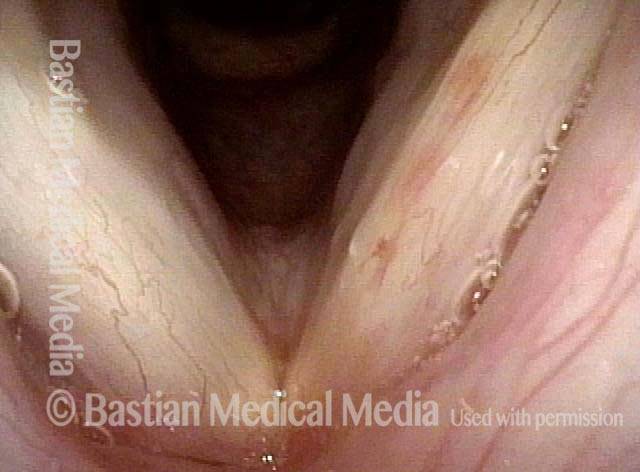
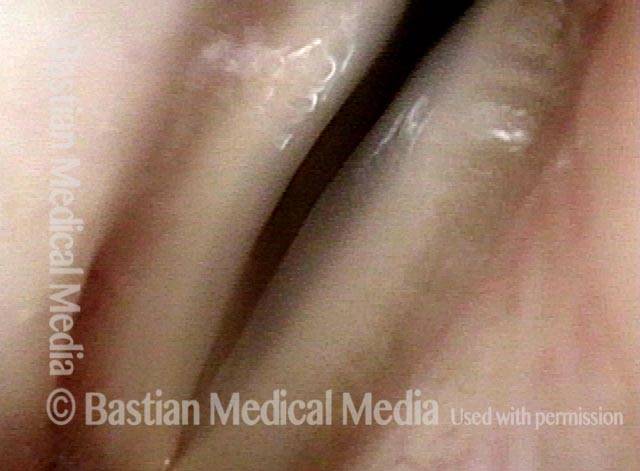
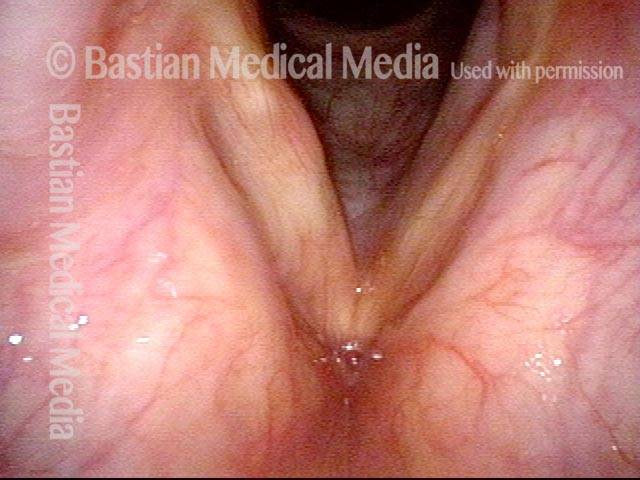
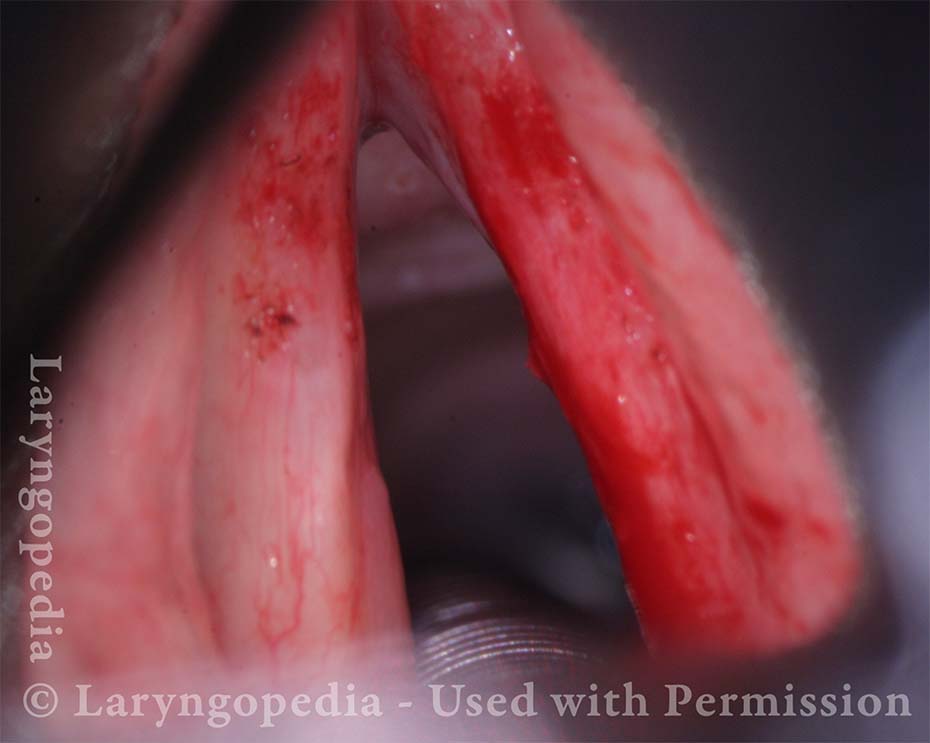
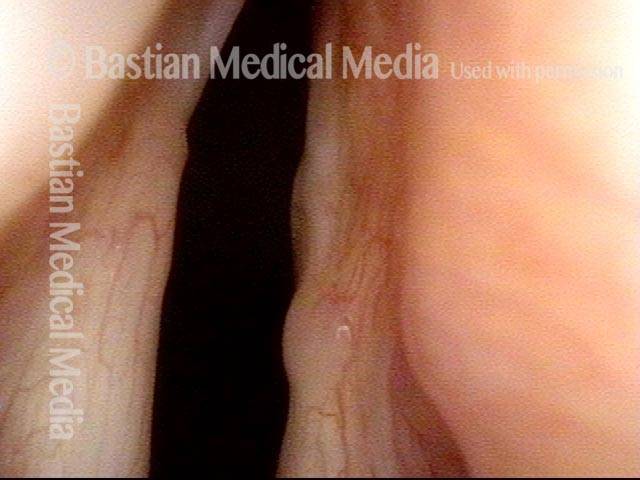
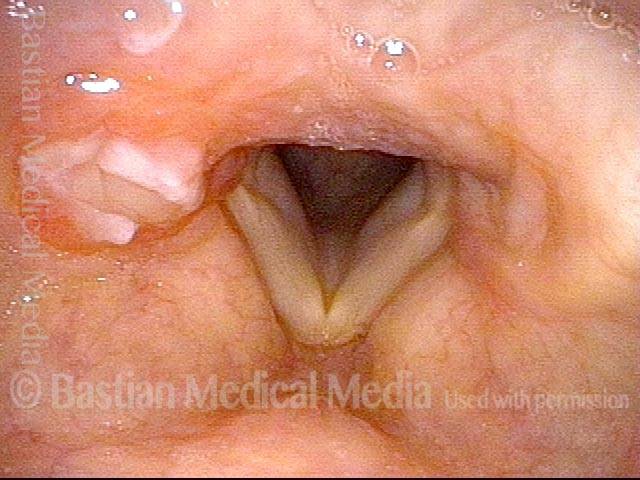
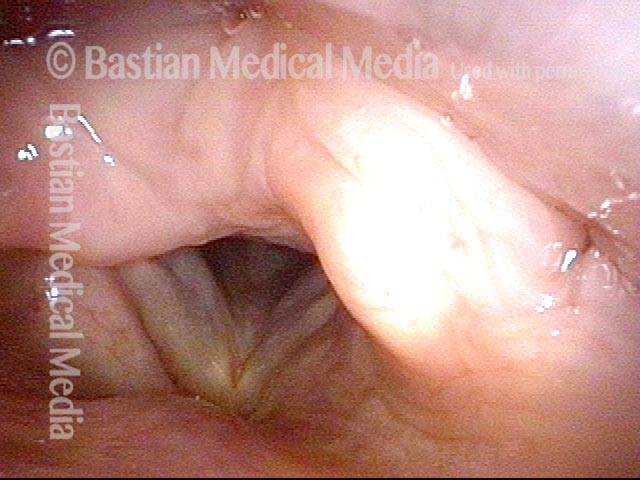
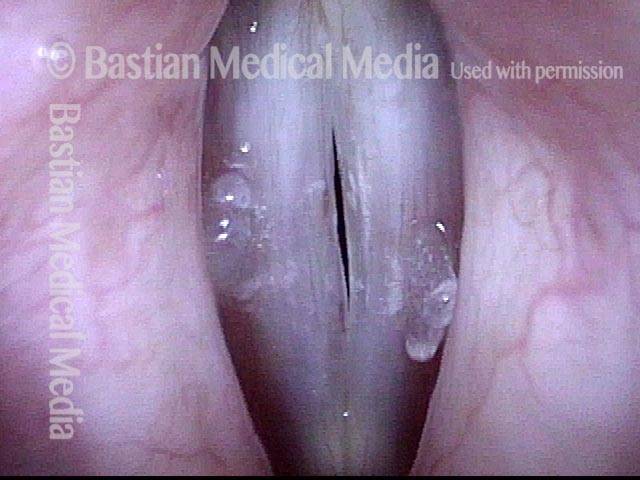
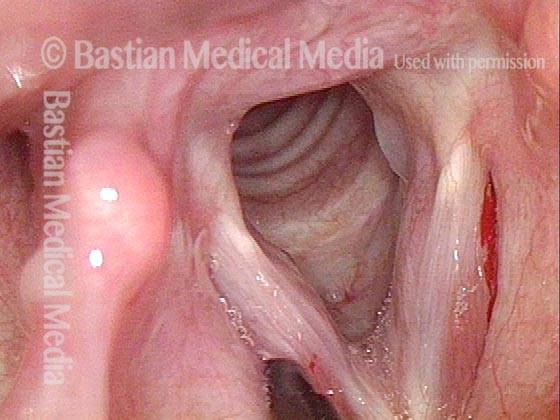
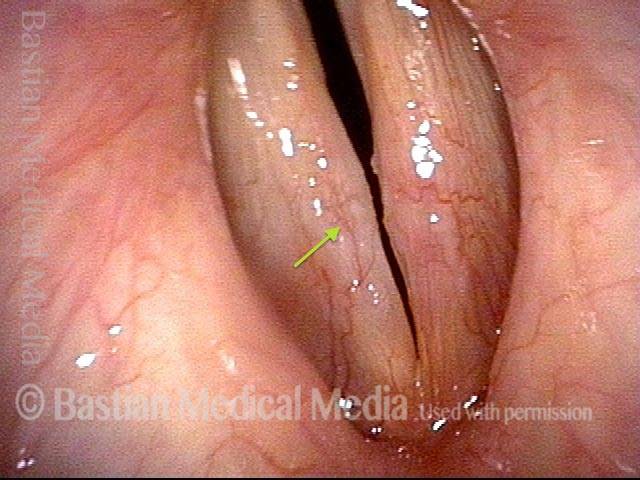
Primary “wound” (6 of 8)
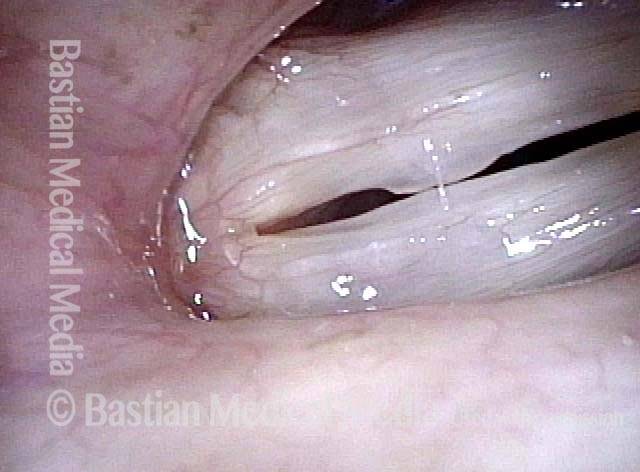
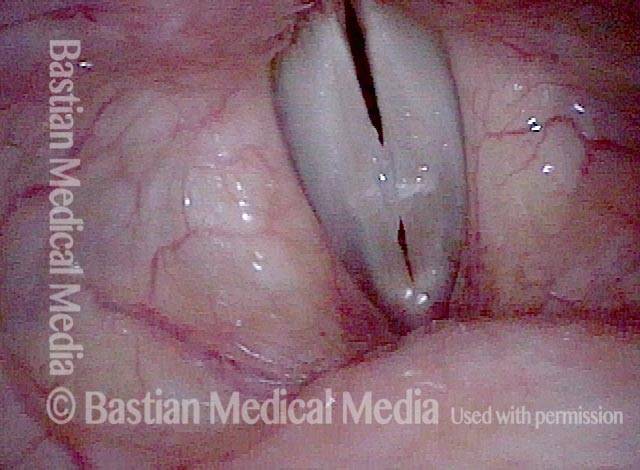
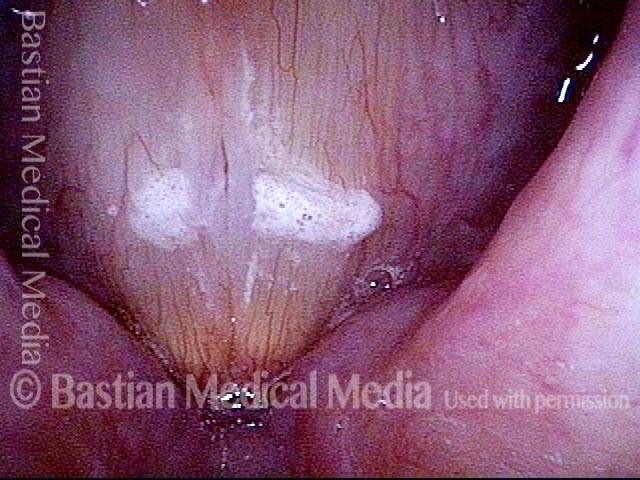
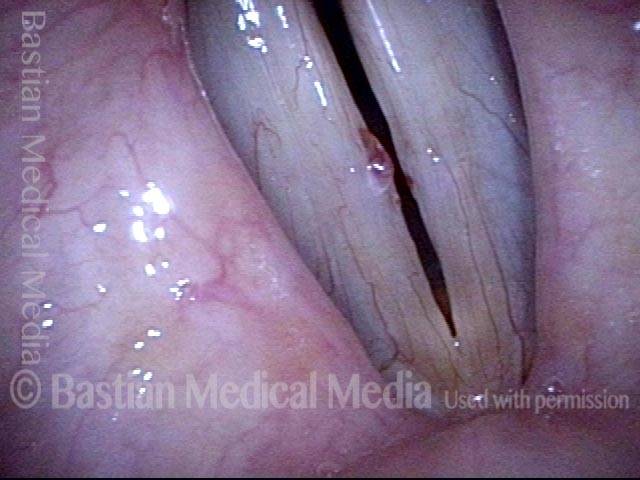
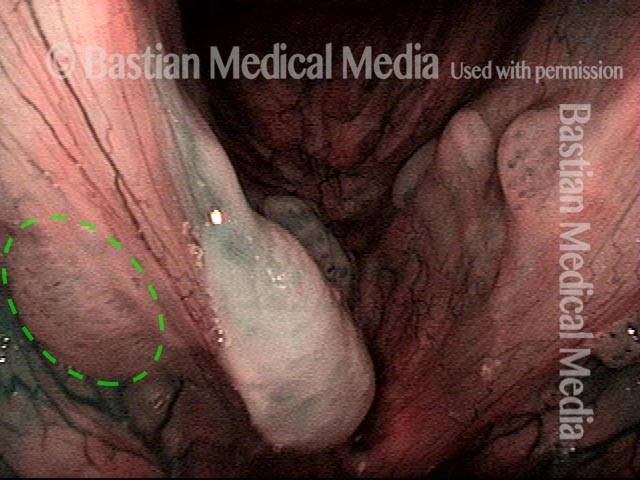
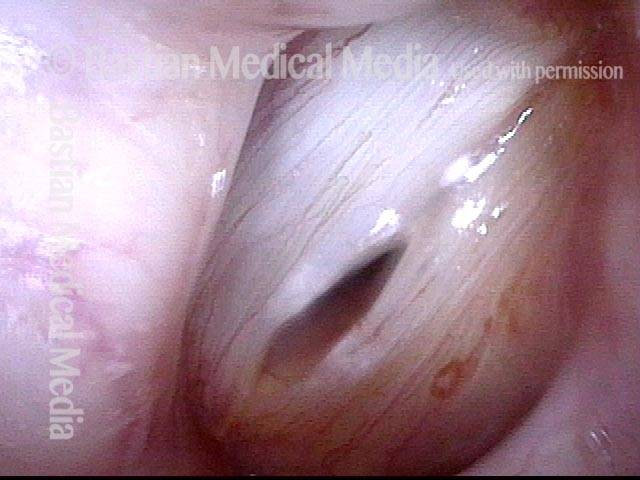
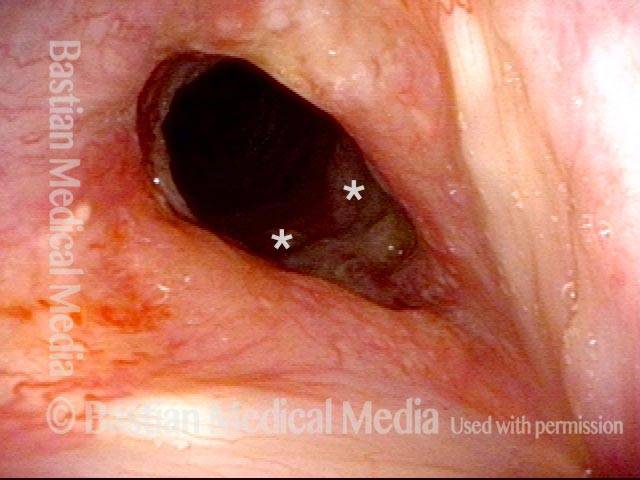
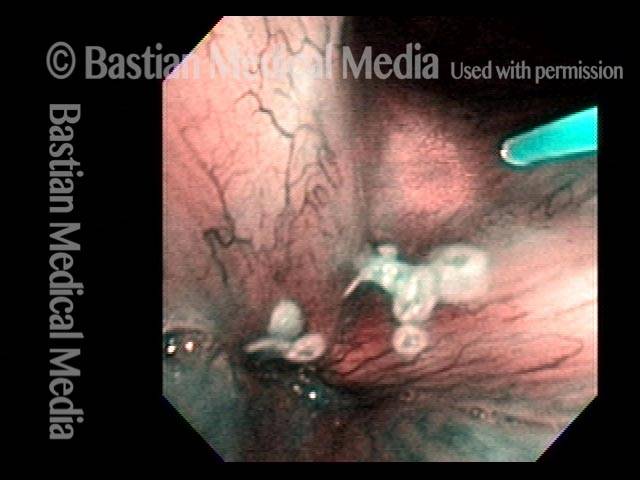
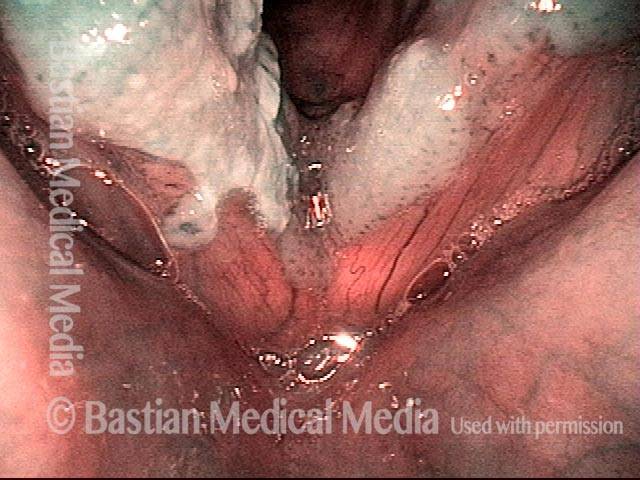
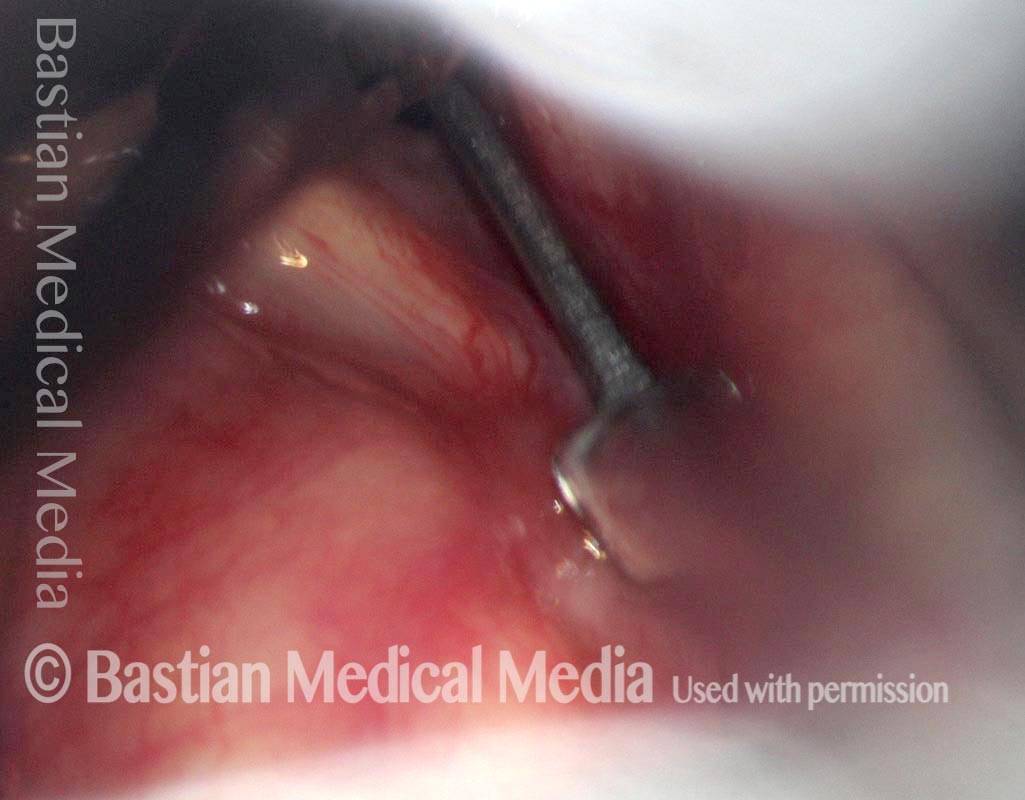
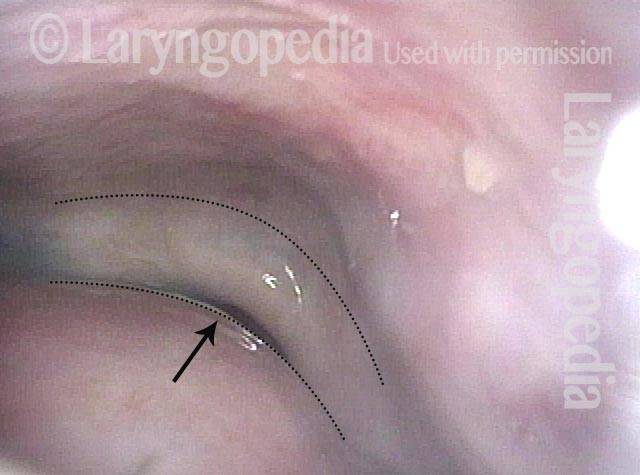
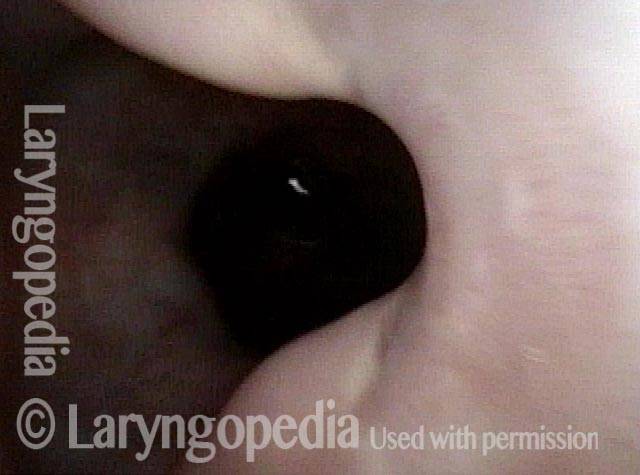
Magnified view. Primary “wound” from polyp removal is at the arrows. Edema of the opposite side is from minimal trimming on that side.
Primary “wound” (6 of 8)
Magnified view. Primary “wound” from polyp removal is at the arrows. Edema of the opposite side is from minimal trimming on that side.
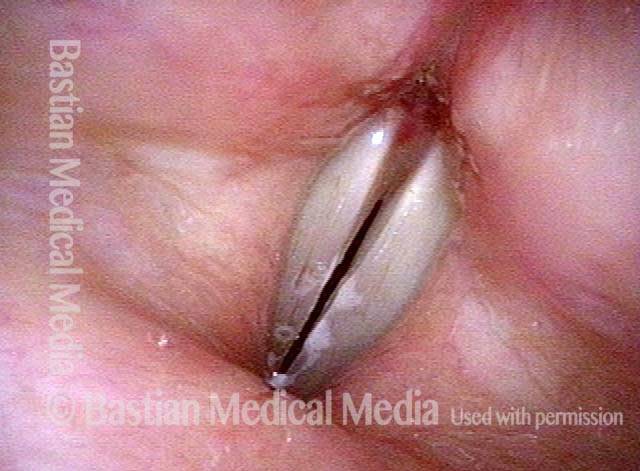
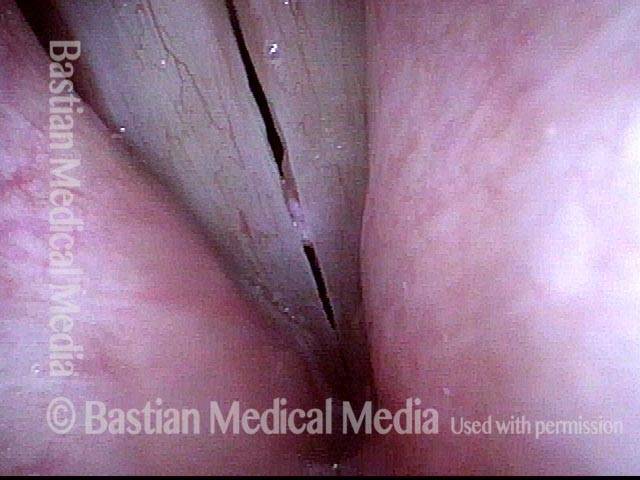
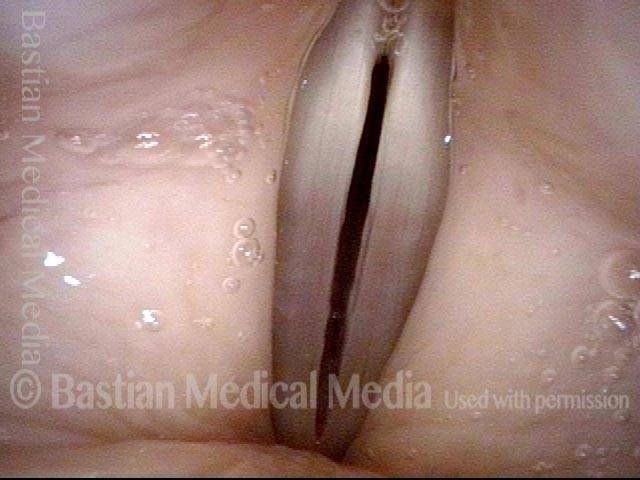
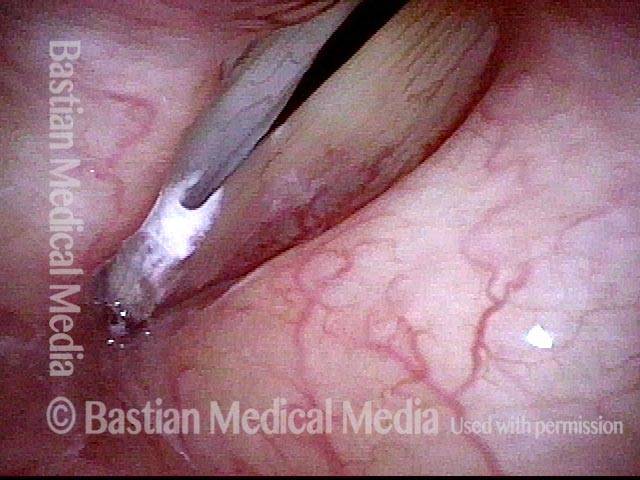
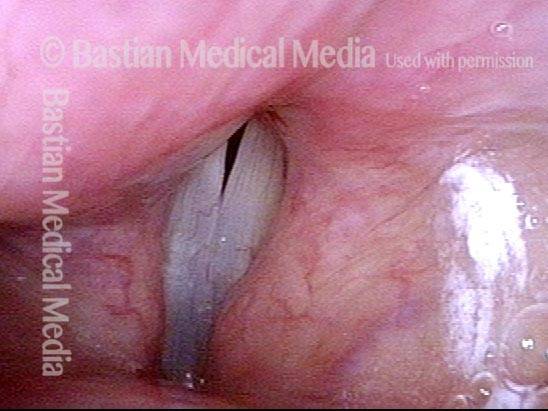
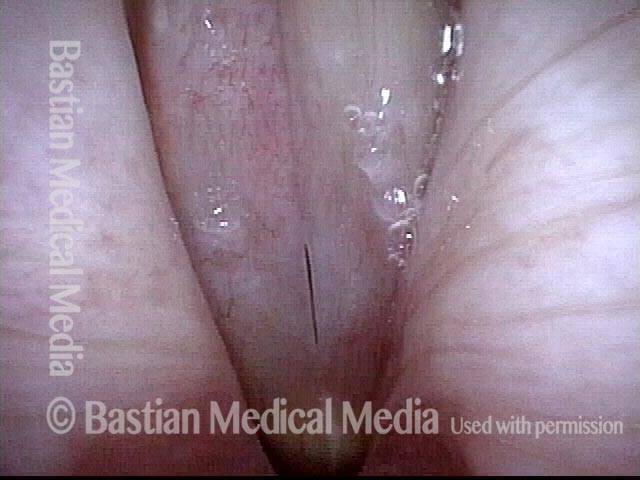
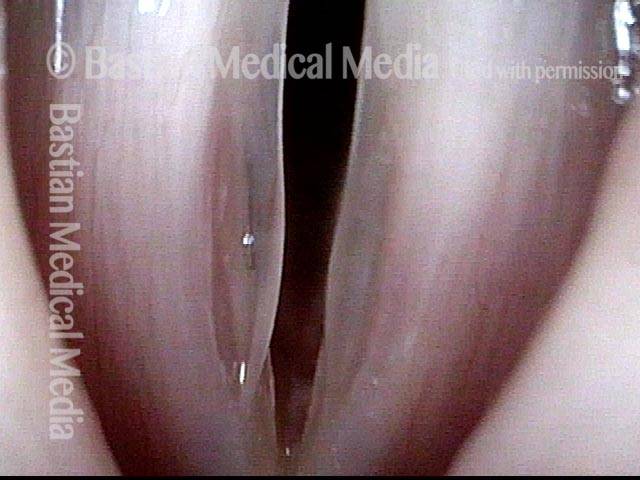
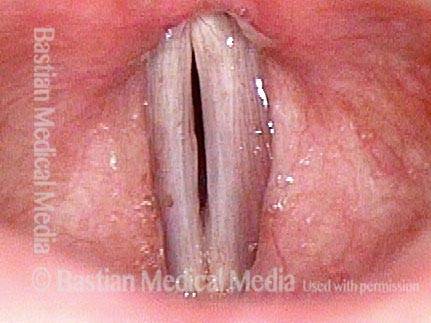
Closed phase (7 of 8)
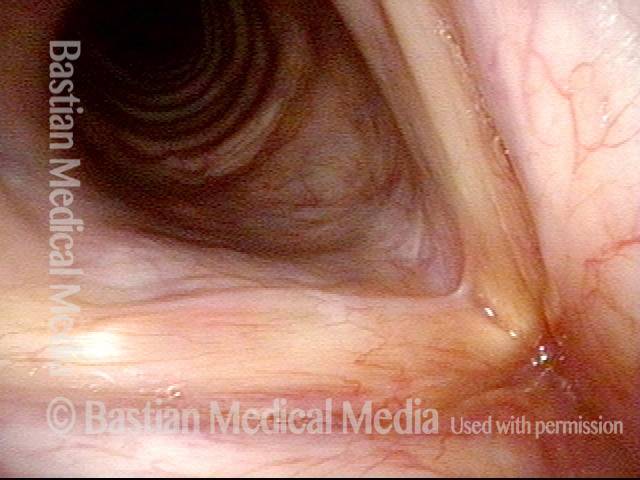
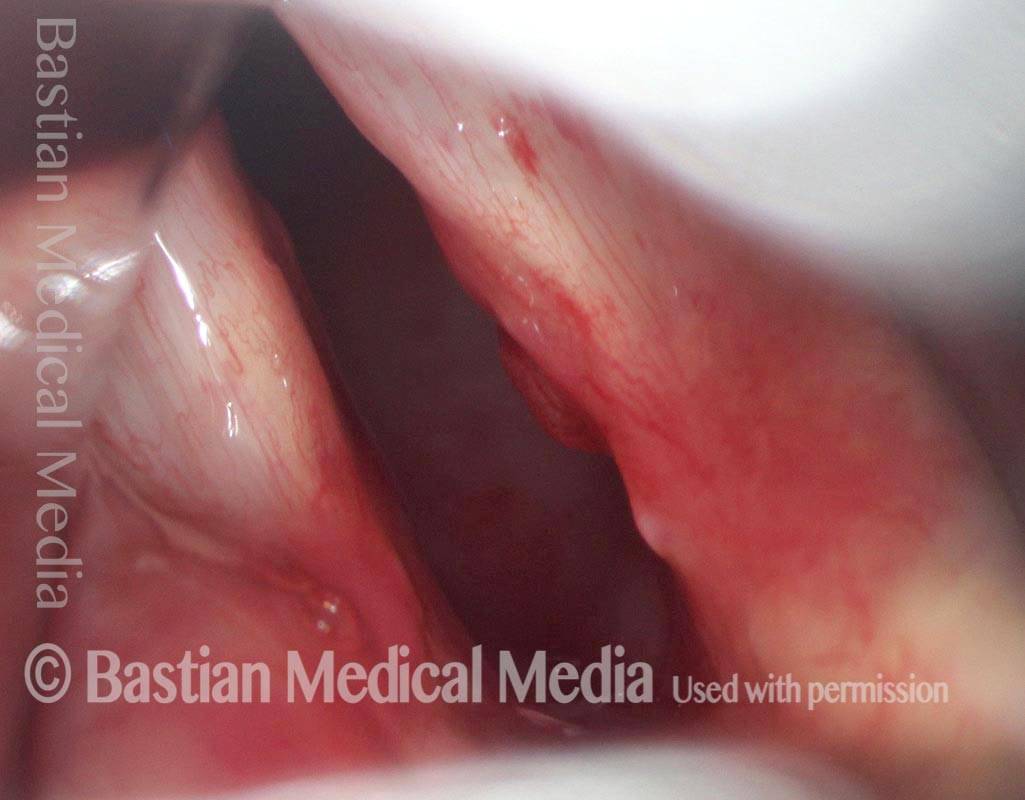
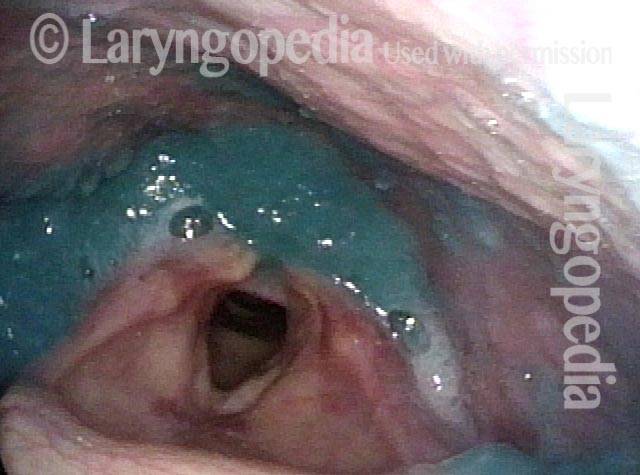
Closed phase of vibration, strobe light, at D5 (587 Hz). Increased mucus is from endotracheal tube and surgical manipulation just hours earlier.
Closed phase (7 of 8)
Closed phase of vibration, strobe light, at D5 (587 Hz). Increased mucus is from endotracheal tube and surgical manipulation just hours earlier.
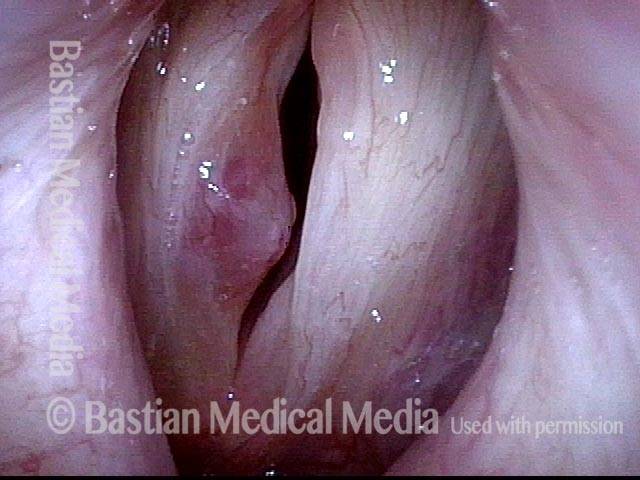
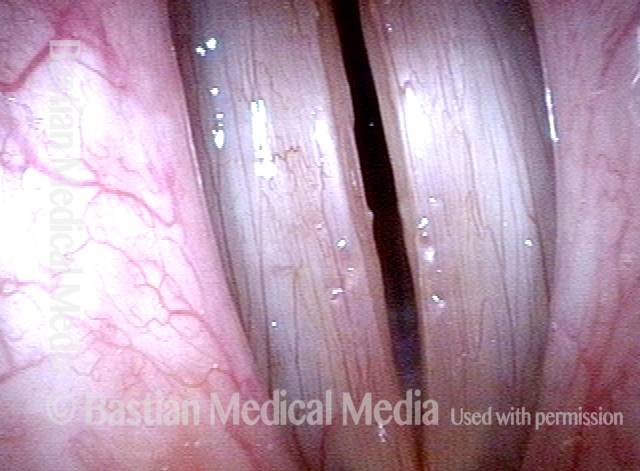
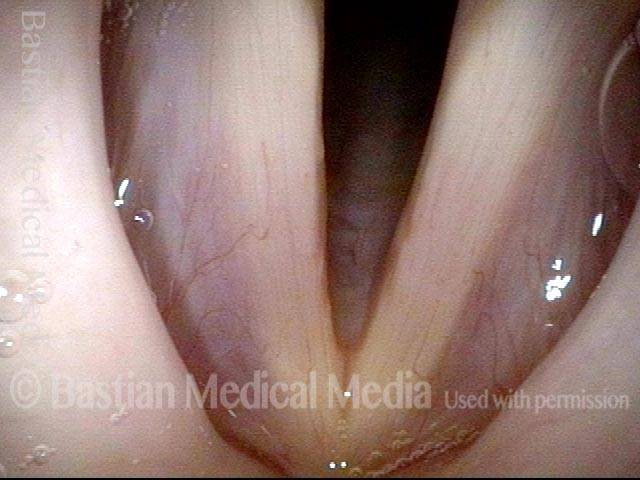
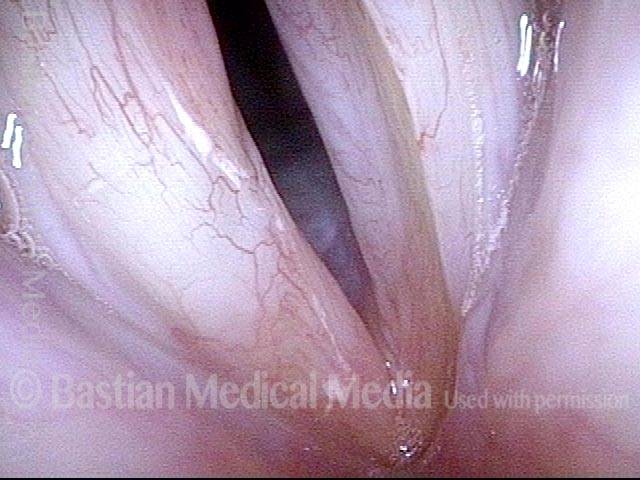
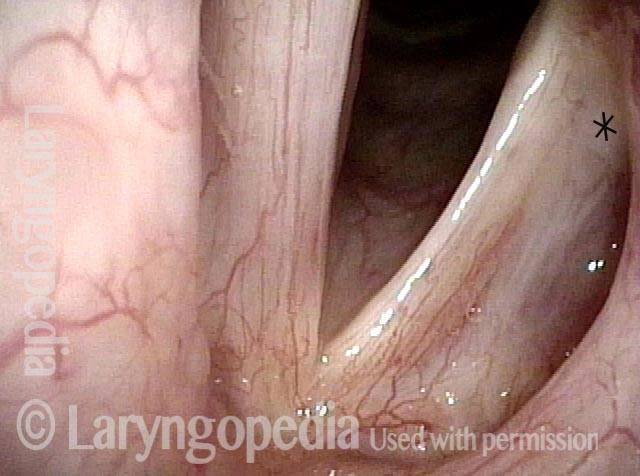
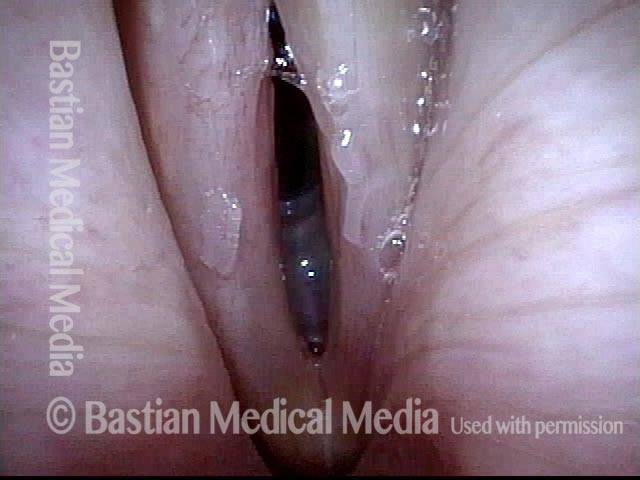
Open phase (8 of 8)
Open phase, at same pitch. Subtle irregularities will “iron out” within over time. Voice is already much better (tested briefly because the patient is within the four days of voice rest, and then will gradually increase amounts of voice use for the subsequent month).
Open phase (8 of 8)
Open phase, at same pitch. Subtle irregularities will “iron out” within over time. Voice is already much better (tested briefly because the patient is within the four days of voice rest, and then will gradually increase amounts of voice use for the subsequent month).
Hemorrhagic Polyp, before and after Surgery
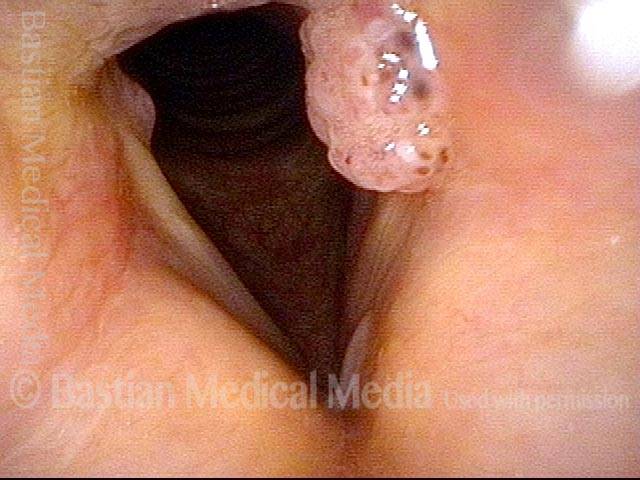
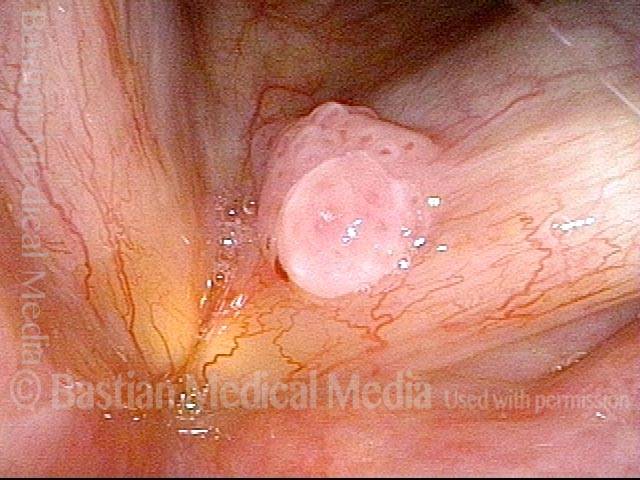
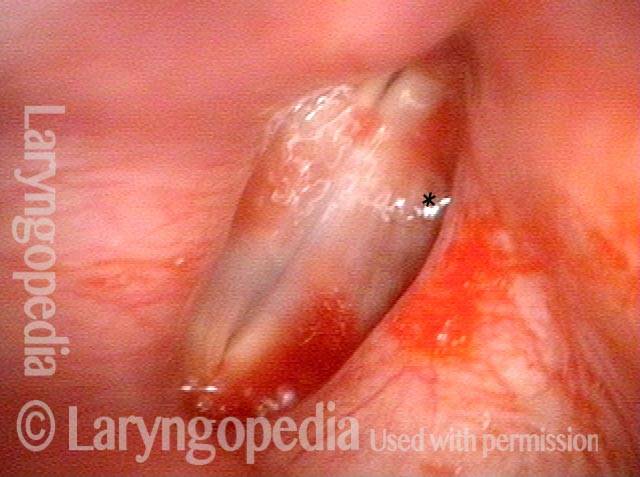
Hemorrhagic polyp (1 of 8)
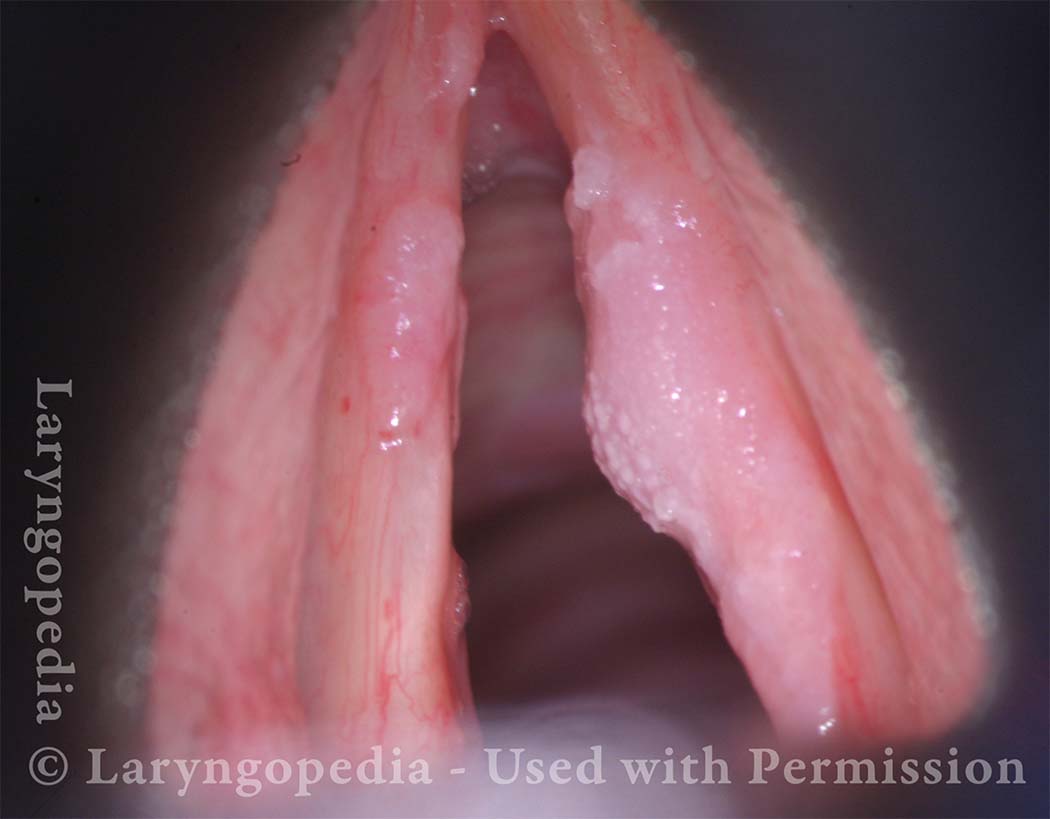
Standard light, close-up view of a hemorrhagic polyp of the vocal cord.
Hemorrhagic polyp (1 of 8)
Standard light, close-up view of a hemorrhagic polyp of the vocal cord.
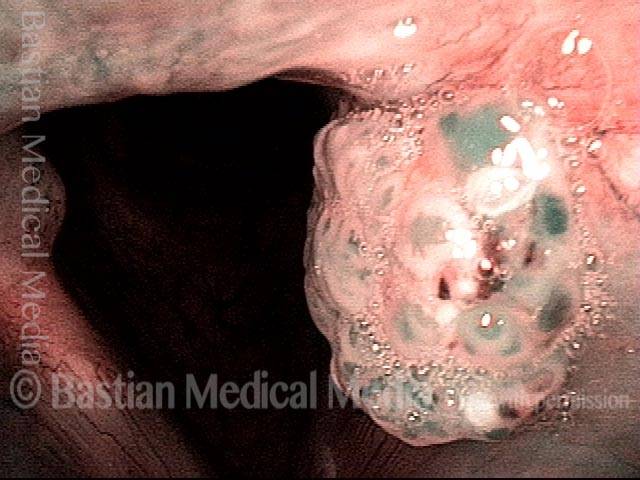
Hemorrhagic polyp (2 of 8)
Strobe light, open phase of vibration.
Hemorrhagic polyp (2 of 8)
Strobe light, open phase of vibration.
Hemorrhagic polyp (3 of 8)
Strobe light, closed phase of vibration.
Hemorrhagic polyp (3 of 8)
Strobe light, closed phase of vibration.
Hemorrhagic polyp: 1 week after surgery (4 of 8)
Same patient, one week after surgical removal of the polyp, standard light.
Hemorrhagic polyp: 1 week after surgery (4 of 8)
Same patient, one week after surgical removal of the polyp, standard light.
Hemorrhagic polyp: 1 week after surgery (5 of 8)
Strobe light, open phase of vibration. Compare with photo 2. Note here that the vibratory amplitude of both cords is the same, showing that the operated cord remains flexible.
Hemorrhagic polyp: 1 week after surgery (5 of 8)
Strobe light, open phase of vibration. Compare with photo 2. Note here that the vibratory amplitude of both cords is the same, showing that the operated cord remains flexible.
Hemorrhagic polyp: 1 week after surgery (6 of 8)
Strobe light, closed phase of vibration. Compare with photo 3; the vocal cords now match much better during voicing, and the voice is completely normalized.
Hemorrhagic polyp: 1 week after surgery (6 of 8)
Strobe light, closed phase of vibration. Compare with photo 3; the vocal cords now match much better during voicing, and the voice is completely normalized.
Hemorrhagic polyp: 7 months after surgery (7 of 8)
Seven months later. Strobe light, closed phase of vibration. The patient feels his voice is normal, and swelling checks don't indicate any impairment.
Hemorrhagic polyp: 7 months after surgery (7 of 8)
Seven months later. Strobe light, closed phase of vibration. The patient feels his voice is normal, and swelling checks don't indicate any impairment.
Hemorrhagic polyp: 7 months after surgery (8 of 8)
Strobe light, open phase of vibration.
Hemorrhagic polyp: 7 months after surgery (8 of 8)
Strobe light, open phase of vibration.
Vocal Polyp, Before and After Surgery
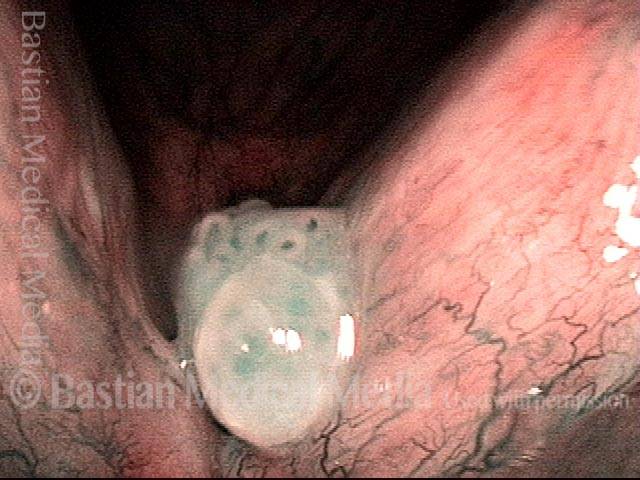
Vocal polyp (1 of 2)
Chronic polyp on the right vocal cord (left of image), with ectatic capillaries, unresponsive to voice rest and therapy.
Vocal polyp (1 of 2)
Chronic polyp on the right vocal cord (left of image), with ectatic capillaries, unresponsive to voice rest and therapy.
Vocal polyp, surgically removed (2 of 2)
Seven weeks after surgical removal and spot coagulation of ectatic capillaries. The margins of the vocal cords now match, and capillaries are normalized. Mucosal vibration is preserved to the highest reaches of the singing range. At this pre-phonatory instant, one can see that muscle memory is keeping the vocal cords slightly apart, suggesting the need for additional speech therapy.
Vocal polyp, surgically removed (2 of 2)
Seven weeks after surgical removal and spot coagulation of ectatic capillaries. The margins of the vocal cords now match, and capillaries are normalized. Mucosal vibration is preserved to the highest reaches of the singing range. At this pre-phonatory instant, one can see that muscle memory is keeping the vocal cords slightly apart, suggesting the need for additional speech therapy.
Vocal polyp (1 of 6)
Prephonatory instant, standard light. The space between the vocal cords is larger than necessary to accommodate the polyp (right of image) and low-profile elevation (left of image).
Vocal polyp (1 of 6)
Prephonatory instant, standard light. The space between the vocal cords is larger than necessary to accommodate the polyp (right of image) and low-profile elevation (left of image).
Vocal polyp (2 of 6)
Phonation with blurring, standard light.
Vocal polyp (2 of 6)
Phonation with blurring, standard light.
Vocal polyp, surgically removed (3 of 6)
Six days after surgical removal. Prephonatory instant, standard light. Compare with photo 1. The patient continues to position vocal cords in a surprisingly separated position, as though the ghosts of the swellings remain. We call this “gap memory” or “posture memory,” though it is a manifestation as well of muscular tension dysphonia.
Vocal polyp, surgically removed (3 of 6)
Six days after surgical removal. Prephonatory instant, standard light. Compare with photo 1. The patient continues to position vocal cords in a surprisingly separated position, as though the ghosts of the swellings remain. We call this “gap memory” or “posture memory,” though it is a manifestation as well of muscular tension dysphonia.
Vocal polyp, surgically removed (4 of 6)
Phonation with blurring, standard light. Compare with photo 2.
Vocal polyp, surgically removed (4 of 6)
Phonation with blurring, standard light. Compare with photo 2.
Vocal polyp, surgically removed (5 of 6)
Phonation, strobe light, open phase of vibration, at high G# (~831 Hz), just below A5. Even at this high pitch, both cords oscillate out to a full lateral excursion.
Vocal polyp, surgically removed (5 of 6)
Phonation, strobe light, open phase of vibration, at high G# (~831 Hz), just below A5. Even at this high pitch, both cords oscillate out to a full lateral excursion.
Vocal polyp, surgically removed (6 of 6)
Phonation, strobe light, closed phase of vibration, also at high G#. Since the patient is only six days postop, mild residual swelling is still present. More importantly, note that this “closed” vibration phase is not in fact fully closed, as further evidence of the patient’s “gap memory” and muscular tension dysphonia. Singing voice-qualified speech therapy and work with a singing teacher will address this.
Vocal polyp, surgically removed (6 of 6)
Phonation, strobe light, closed phase of vibration, also at high G#. Since the patient is only six days postop, mild residual swelling is still present. More importantly, note that this “closed” vibration phase is not in fact fully closed, as further evidence of the patient’s “gap memory” and muscular tension dysphonia. Singing voice-qualified speech therapy and work with a singing teacher will address this.
Vocal polyp (1 of 6)
An operatic baritone has a chronic left vocal cord polyp (right of image), and small contact reaction, right cord.
Vocal polyp (1 of 6)
An operatic baritone has a chronic left vocal cord polyp (right of image), and small contact reaction, right cord.
Vocal polyp (2 of 6)
Phonation, open phase of vibration, upper middle voice, showing obvious margin elevation of the left cord (right of image). Voice is hoarse.
Vocal polyp (2 of 6)
Phonation, open phase of vibration, upper middle voice, showing obvious margin elevation of the left cord (right of image). Voice is hoarse.
Vocal polyp (3 of 6)
Maximum closed phase of vibration, showing polyp-induced gap, causing air wasting and hoarse voice quality.
Vocal polyp (3 of 6)
Maximum closed phase of vibration, showing polyp-induced gap, causing air wasting and hoarse voice quality.
Vocal polyp, surgically removed (4 of 6)
Sixth day after microlaryngoscopic removal of the polyp. Note the red, 2-millimeter “wound” where the polyp was removed.
Vocal polyp, surgically removed (4 of 6)
Sixth day after microlaryngoscopic removal of the polyp. Note the red, 2-millimeter “wound” where the polyp was removed.
Vocal polyp, surgically removed (5 of 6)
At extremely high falsetto, open phase of vibration, showing uniform width of glottic chink. Voice is normal, even at this revealing, high pitch.
Vocal polyp, surgically removed (5 of 6)
At extremely high falsetto, open phase of vibration, showing uniform width of glottic chink. Voice is normal, even at this revealing, high pitch.
Vocal polyp, surgically removed (6 of 6)
Closed phase of vibration, high falsetto, shows equal vibratory amplitude on both sides (no stiffness) and excellent match of the cords.
Vocal polyp, surgically removed (6 of 6)
Closed phase of vibration, high falsetto, shows equal vibratory amplitude on both sides (no stiffness) and excellent match of the cords.
Pre-op and very early Post-op Mucosal Match and Flexibility in Male Singer
Bilateral chronic injuries (1 of 8)
Young music teacher and choral director with chronic hoarseness for more than a year. Note bilateral chronic injuries, and also recent bruise of the right cord (left of photo).
Bilateral chronic injuries (1 of 8)
Young music teacher and choral director with chronic hoarseness for more than a year. Note bilateral chronic injuries, and also recent bruise of the right cord (left of photo).
Closeup of injuries (2 of 8)
Closeup of injuries and their "refusal" to let the cords approximate, when attempting (unsuccessfully) to sing a high pitch.
Closeup of injuries (2 of 8)
Closeup of injuries and their "refusal" to let the cords approximate, when attempting (unsuccessfully) to sing a high pitch.
Post microsurgery (3 of 8)
7 days after vocal cord microsurgery, the voice can already pass for normal quality and capability. Compare with photo 1.
Post microsurgery (3 of 8)
7 days after vocal cord microsurgery, the voice can already pass for normal quality and capability. Compare with photo 1.
Prephonatory instant (4 of 8)
Prephonatory instant at high pitch, showing that the match of the cords is already markedly restored. The tiny elevation will disappear with further healing.
Prephonatory instant (4 of 8)
Prephonatory instant at high pitch, showing that the match of the cords is already markedly restored. The tiny elevation will disappear with further healing.
Closed phase, A-flat 3 (5 of 8)
Closed phase of vibration at A-flat 3 (208 Hz), seen under strobe light.
Closed phase, A-flat 3 (5 of 8)
Closed phase of vibration at A-flat 3 (208 Hz), seen under strobe light.
Open phase (6 of 8)
This open phase shows what is equally important: that both cords display equal vibratory flexibility; that is, there is no stiffness or scarring.
Open phase (6 of 8)
This open phase shows what is equally important: that both cords display equal vibratory flexibility; that is, there is no stiffness or scarring.
Dramatically improved match (7 of 8)
This man also has clear, normal falsetto voice: closed phase of vibration at A-flat 4 (415 Hz), again showing dramatically improved match.
Dramatically improved match (7 of 8)
This man also has clear, normal falsetto voice: closed phase of vibration at A-flat 4 (415 Hz), again showing dramatically improved match.
Equal mucosal flexibility (8 of 8)
Again more important is evidence of equal mucosal flexibility between the two sides, as seen in this open phase of vibration, at the same pitch.
Equal mucosal flexibility (8 of 8)
Again more important is evidence of equal mucosal flexibility between the two sides, as seen in this open phase of vibration, at the same pitch.
For more info: Vocal polyps
Vocal Nodules:
Fibrosis as a Base to Nodules, Before and After Surgery
Bilateral polypoid nodules (1 of 8)
Fibrosis as a Base to Nodules, Before and After Surgery
Bilateral polypoid nodules (1 of 8)
Fibrosis as a Base to Nodules, Before and After Surgery
Narrow-band lighting (2 of 8)
At greater magnification, and also under narrow-band light. The area of fibrosis is more clearly seen, now without the dotted lines.
Narrow-band lighting (2 of 8)
At greater magnification, and also under narrow-band light. The area of fibrosis is more clearly seen, now without the dotted lines.
Closed phase (3 of 8)
Closed phase of vibration at ~A4 (440 Hz), as seen under strobe light.
Closed phase (3 of 8)
Closed phase of vibration at ~A4 (440 Hz), as seen under strobe light.
Open phase (4 of 8)
Open phase of vibration also at ~ A4.
Open phase (4 of 8)
Open phase of vibration also at ~ A4.
Two weeks after surgery (5 of 8)
Less than two weeks after surgical removal of the polyps. The faint white zone of margin fibrosis is again seen. Compare with photo 1.
Two weeks after surgery (5 of 8)
Less than two weeks after surgical removal of the polyps. The faint white zone of margin fibrosis is again seen. Compare with photo 1.
Phonation (6 of 8)
Phonation under standard light shows that vocal cord margins now match, and both margins blur; suggesting vibratory flexibility.
Phonation (6 of 8)
Phonation under standard light shows that vocal cord margins now match, and both margins blur; suggesting vibratory flexibility.
Margin fibrosis (7 of 8)
Closed phase of vibration, at ~ A4 (440 Hz), as seen under strobe light. Margin fibrosis seen best here, indicated by the black dotted line. Compare with photo 3.
Margin fibrosis (7 of 8)
Closed phase of vibration, at ~ A4 (440 Hz), as seen under strobe light. Margin fibrosis seen best here, indicated by the black dotted line. Compare with photo 3.
Open phase (8 of 8)
Open phase of vibration. The same pitch (A4) reveals excellent vibratory flexibility and equal amplitude (lateral excursion) of vibration. Compare with photo 4.
Open phase (8 of 8)
Open phase of vibration. The same pitch (A4) reveals excellent vibratory flexibility and equal amplitude (lateral excursion) of vibration. Compare with photo 4.
Vocal Nodules, Before and After Surgery
Vocal nodules (1 of 6)
Strobe light, phonation, open phase of vibration, at the pitch D5 (~587 Hz). There are vocal nodules on both vocal cords, of very long duration, even after voice rest and speech therapy. Compare with photos 3 and 5.
Vocal nodules (1 of 6)
Strobe light, phonation, open phase of vibration, at the pitch D5 (~587 Hz). There are vocal nodules on both vocal cords, of very long duration, even after voice rest and speech therapy. Compare with photos 3 and 5.
Breathy voice (2 of 6)
Same as photo 1, but during the closed phase of vibration. The nodules keep the vocal cords from coming together completely (as seen here), making the patient’s voice breathy. Compare with photos 4 and 6.
Breathy voice (2 of 6)
Same as photo 1, but during the closed phase of vibration. The nodules keep the vocal cords from coming together completely (as seen here), making the patient’s voice breathy. Compare with photos 4 and 6.
1 week after surgery (3 of 6)
One week after surgical removal of the vocal nodules. Strobe light, phonation, open phase of vibration, at the pitch B5 (~988 Hz). (The small “blob” seen at the midpoint of the cords is just incidental mucus.)
1 week after surgery (3 of 6)
One week after surgical removal of the vocal nodules. Strobe light, phonation, open phase of vibration, at the pitch B5 (~988 Hz). (The small “blob” seen at the midpoint of the cords is just incidental mucus.)
Closed phase (4 of 6)
Same as photo 3, but during the closed phase of vibration.
Closed phase (4 of 6)
Same as photo 3, but during the closed phase of vibration.
7 weeks after surgery (5 of 6)
Seven weeks after surgical removal of the nodules. Strobe light, phonation, open phase of vibration, at the pitch C#6 (~1109 Hz). (Incidental mucus is obscuring the posterior end of the vocal cords.)
7 weeks after surgery (5 of 6)
Seven weeks after surgical removal of the nodules. Strobe light, phonation, open phase of vibration, at the pitch C#6 (~1109 Hz). (Incidental mucus is obscuring the posterior end of the vocal cords.)
7 weeks after surgery (6 of 6)
Same as photo 5, but during the closed phase of vibration. Voice is no longer breathy, and the upper range has been restored.
7 weeks after surgery (6 of 6)
Same as photo 5, but during the closed phase of vibration. Voice is no longer breathy, and the upper range has been restored.
Vocal nodules (1 of 10)
Vocal nodules under standard light. Note asymmetry in size.
Vocal nodules (1 of 10)
Vocal nodules under standard light. Note asymmetry in size.
Prephonatory instant (2 of 10)
Prephonatory instant, standard light.
Prephonatory instant (2 of 10)
Prephonatory instant, standard light.
Translucency (3 of 10)
Closed phase of vibration, with notable translucency of the right vocal cord (left of image), which is often a predictor of chronicity and only partial response to speech (voice) therapy.
Translucency (3 of 10)
Closed phase of vibration, with notable translucency of the right vocal cord (left of image), which is often a predictor of chronicity and only partial response to speech (voice) therapy.
Open phase (4 of 10)
Open phase of vibration, strobe light.
Open phase (4 of 10)
Open phase of vibration, strobe light.
1 week after surgery (5 of 10)
A week after vocal cord microsurgery, standard light.
1 week after surgery (5 of 10)
A week after vocal cord microsurgery, standard light.
1 week after surgery (6 of 10)
Open phase of vibration, strobe light.
1 week after surgery (6 of 10)
Open phase of vibration, strobe light.
1 week after surgery (7 of 10)
Closed phase of vibration, strobe light, showing tiny margin elevations, bilaterally.
1 week after surgery (7 of 10)
Closed phase of vibration, strobe light, showing tiny margin elevations, bilaterally.
Vocal nodules: 10 weeks after surgery (8 of 10)
Prephonatory instant shows recurrent swelling due to persistent vocal overuse, despite careful preoperative preparation for surgery by a voice-qualified speech pathologist. Patients must know “we are only operating on your vocal cords, not your personality, occupation, friend group, social life, etc.”
Vocal nodules: 10 weeks after surgery (8 of 10)
Prephonatory instant shows recurrent swelling due to persistent vocal overuse, despite careful preoperative preparation for surgery by a voice-qualified speech pathologist. Patients must know “we are only operating on your vocal cords, not your personality, occupation, friend group, social life, etc.”
10 weeks after surgery (9 of 10)
Open phase of vibration, strobe light.
10 weeks after surgery (9 of 10)
Open phase of vibration, strobe light.
10 weeks after surgery (10 of 10)
Closed phase of vibration, strobe light. The “original equipment” capabilities of the voice early after successful vocal cord microsurgery (above) have been diminished, but capabilities remain markedly better than they were with the original lesions.
10 weeks after surgery (10 of 10)
Closed phase of vibration, strobe light. The “original equipment” capabilities of the voice early after successful vocal cord microsurgery (above) have been diminished, but capabilities remain markedly better than they were with the original lesions.
Vocal nodules (1 of 4)
Vocal nodules, moderately large, seen with cords in abducted (breathing) position.
Vocal nodules (1 of 4)
Vocal nodules, moderately large, seen with cords in abducted (breathing) position.
Phonation (2 of 4)
Phonation, showing early contact of the nodules, and large gaps anterior and posterior to the nodules.
Phonation (2 of 4)
Phonation, showing early contact of the nodules, and large gaps anterior and posterior to the nodules.
After surgery (3 of 4)
Phonatory position, after surgical removal. Note the straightened vocal cord margins.
After surgery (3 of 4)
Phonatory position, after surgical removal. Note the straightened vocal cord margins.
After surgery (4 of 4)
Breathing position, also post-surgery.
After surgery (4 of 4)
Breathing position, also post-surgery.
Pre-and 1 Week Post-removal Vocal Nodules
Vocal nodules (1 of 8)
Semi-professional high soprano with grossly impaired upper voice due to polypoid (fusiform) vocal nodule.
Vocal nodules (1 of 8)
Semi-professional high soprano with grossly impaired upper voice due to polypoid (fusiform) vocal nodule.
Muscular tension dysphonia (2 of 8)
Phonatory view shows a degree of muscular tension dysphonia (separated vocal processes), too.
Muscular tension dysphonia (2 of 8)
Phonatory view shows a degree of muscular tension dysphonia (separated vocal processes), too.
Open phase (3 of 8)
Nearly open phase under strobe light at B4 (494 Hz).
Open phase (3 of 8)
Nearly open phase under strobe light at B4 (494 Hz).
Closed phase (4 of 8)
Closed phase of vibration, aslo at B4.
Closed phase (4 of 8)
Closed phase of vibration, aslo at B4.
Post-op, one week (5 of 8)
A week after surgical removal of the nodules, at the prephonatory instant, D5, showing margin irregularity.
Post-op, one week (5 of 8)
A week after surgical removal of the nodules, at the prephonatory instant, D5, showing margin irregularity.
Phonation (6 of 8)
Phonation, with vibratory blur under standard light.
Phonation (6 of 8)
Phonation, with vibratory blur under standard light.
Open phase (7 of 8)
Open phase of vibration (strobe light), at D5 (587 Hz). Irregular margins will iron out across time.
Open phase (7 of 8)
Open phase of vibration (strobe light), at D5 (587 Hz). Irregular margins will iron out across time.
Closed phase (8 of 8)
At closed phase of vibration, also at D5. Note excellent match, bilaterally equal vibratory excursions, and partial correction of the MTD posterior commissure gap.
Closed phase (8 of 8)
At closed phase of vibration, also at D5. Note excellent match, bilaterally equal vibratory excursions, and partial correction of the MTD posterior commissure gap.
Vocal Nodule Postop Irregularity yet Match and Flexibility
Large vocal nodules (1 of 8)
Bilateral large vocal nodules in band singer that does close harmony musical styles.
Large vocal nodules (1 of 8)
Bilateral large vocal nodules in band singer that does close harmony musical styles.
Narrow band light (2 of 8)
Now under narrow band light to accentuate the vascular pattern.
Narrow band light (2 of 8)
Now under narrow band light to accentuate the vascular pattern.
One week after surgery (5 of 8)
A week after surgery, the “wounds” measure about 3mm long (at arrows).
One week after surgery (5 of 8)
A week after surgery, the “wounds” measure about 3mm long (at arrows).
Prephonatory instant (6 of 8)
Prephonatory instant, standard light, at C#5 (554 Hz).
Prephonatory instant (6 of 8)
Prephonatory instant, standard light, at C#5 (554 Hz).
Open phase (7 of 8)
Open phase of vibration at E5 (659 Hz). Voice is markedly improved.
Open phase (7 of 8)
Open phase of vibration at E5 (659 Hz). Voice is markedly improved.
For more info: Vocal nodules
Cancer:
Vocal Cord Cancer, before and after Surgery
Vocal cord cancer (1 of 4)
Squamous cell carcinoma, right vocal cord (left of image), standard light.
Vocal cord cancer (1 of 4)
Squamous cell carcinoma, right vocal cord (left of image), standard light.
Vocal cord cancer, 1 week after surgery (2 of 4)
One week after laser excision. See irregular granulation especially at lower margin of excision.
Vocal cord cancer, 1 week after surgery (2 of 4)
One week after laser excision. See irregular granulation especially at lower margin of excision.
Vocal cord cancer, 1 month after surgery (3 of 4)
Approximately one month after excision, healing progressing.
Vocal cord cancer, 1 month after surgery (3 of 4)
Approximately one month after excision, healing progressing.
Vocal cord cancer, after complete healing (4 of 4)
After complete healing, patient has a voice that passes for normal. Under strobe light, right cord oscillates well except at very high vocal pitch. Note, however, the mild pseudo-bowing of the right cord due to tissue loss, and that there is a mucosal wave on the left, but not on the right.
Vocal cord cancer, after complete healing (4 of 4)
After complete healing, patient has a voice that passes for normal. Under strobe light, right cord oscillates well except at very high vocal pitch. Note, however, the mild pseudo-bowing of the right cord due to tissue loss, and that there is a mucosal wave on the left, but not on the right.
Vocal cord cancer (1 of 8)
Patient from elsewhere, first seen 9 months after radiotherapy, with obvious persistent right vocal cord cancer.
Vocal cord cancer (1 of 8)
Patient from elsewhere, first seen 9 months after radiotherapy, with obvious persistent right vocal cord cancer.
Vocal cord cancer (2 of 8)
Closer view, during phonation, showing deep ulceration and rolled upper and lower border of cancer.
Vocal cord cancer (2 of 8)
Closer view, during phonation, showing deep ulceration and rolled upper and lower border of cancer.
Vocal cord cancer, 1 week after surgery (3 of 8)
One week after aggressive cordectomy, right, including down to inner perichondrium of thyroid cartilage.
Vocal cord cancer, 1 week after surgery (3 of 8)
One week after aggressive cordectomy, right, including down to inner perichondrium of thyroid cartilage.
Vocal cord cancer, 1 week after surgery (4 of 8)
Phonation, showing that the left vocal cord now has no “partner” against which to vibrate, and this explains the marked breathiness.
Vocal cord cancer, 1 week after surgery (4 of 8)
Phonation, showing that the left vocal cord now has no “partner” against which to vibrate, and this explains the marked breathiness.
Vocal cord cancer, 7 weeks after surgery (5 of 8)
Nearly complete healing after complete cordectomy right vocal cord. Only residual granulation.
Vocal cord cancer, 7 weeks after surgery (5 of 8)
Nearly complete healing after complete cordectomy right vocal cord. Only residual granulation.
Vocal cord cancer, 7 weeks after surgery (6 of 8)
Closer view of defect. Thin mucosa covers inner surface of thyroid cartilage, and residual exposed cartilage, not yet healed over with mucosa, at arrow.
Vocal cord cancer, 7 weeks after surgery (6 of 8)
Closer view of defect. Thin mucosa covers inner surface of thyroid cartilage, and residual exposed cartilage, not yet healed over with mucosa, at arrow.
Vocal cord cancer, 7 weeks after surgery (7 of 8)
At maximum phonatory adduction. Note that the left vocal process is turned medially (arrow), signifying maximum adductory “effort” of that side. There is no right vocal cord, and hence there is no possibility of glottic voice.
Vocal cord cancer, 7 weeks after surgery (7 of 8)
At maximum phonatory adduction. Note that the left vocal process is turned medially (arrow), signifying maximum adductory “effort” of that side. There is no right vocal cord, and hence there is no possibility of glottic voice.
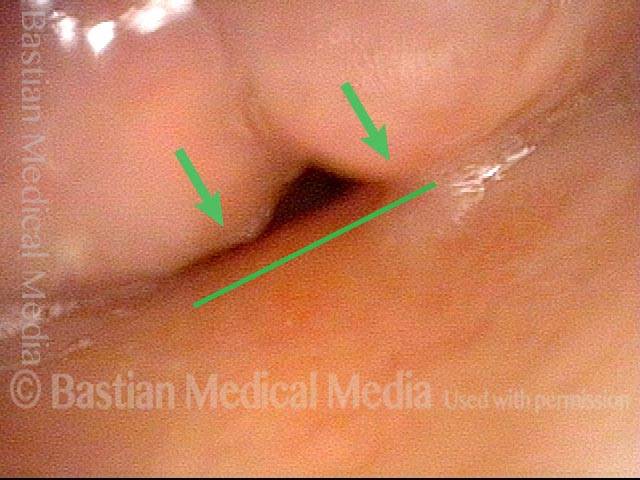
Vocal cord cancer, 7 weeks after surgery (8 of 8)
Vibration of the arytenoid apices (arrows) against the petiole of the epiglottis (line), providing a rough, voice serviceable for quiet conversation, but highly limited in noisy surroundings.
Vocal cord cancer, 7 weeks after surgery (8 of 8)
Vibration of the arytenoid apices (arrows) against the petiole of the epiglottis (line), providing a rough, voice serviceable for quiet conversation, but highly limited in noisy surroundings.
Scarring after Cancer Treatment but with very Good Voice
Post laser excision (1 of 4)
Ten years after laser excision of a left vocal cord (right of photo) cancer, viewed from a distance. Voice has been extremely serviceable, if slightly husky.
Post laser excision (1 of 4)
Ten years after laser excision of a left vocal cord (right of photo) cancer, viewed from a distance. Voice has been extremely serviceable, if slightly husky.
Prephonatory instant (2 of 4)
Closer visualization at the prephonatory instant. Now the pseudobowing of the left cord (from tissue loss is easily seen.
Prephonatory instant (2 of 4)
Closer visualization at the prephonatory instant. Now the pseudobowing of the left cord (from tissue loss is easily seen.
Phonation (3 of 4)
Making voice, the faint blurring of the right cord margin (left of photo), but non-vibrating left cord (right of photo) can be more easily appreciated.
Phonation (3 of 4)
Making voice, the faint blurring of the right cord margin (left of photo), but non-vibrating left cord (right of photo) can be more easily appreciated.
Close-up view (4 of 4)
At very close range, medial-to-lateral capillary reorientation—typical of superficial cordectomy after healing.
Close-up view (4 of 4)
At very close range, medial-to-lateral capillary reorientation—typical of superficial cordectomy after healing.
Vocal Cord Cancer, before, during, and after Radiation
Vocal cord cancer (1 of 8)
Superficial cancer involving both vocal cords. This is stage 1 disease (T1B). The greatest bulk is on the right posterior cord (left of image), but the majority of both cords is involved with at least superficial disease. A faint dotted rectangle indicates the zoomed-in area seen in photo 2.
Vocal cord cancer (1 of 8)
Superficial cancer involving both vocal cords. This is stage 1 disease (T1B). The greatest bulk is on the right posterior cord (left of image), but the majority of both cords is involved with at least superficial disease. A faint dotted rectangle indicates the zoomed-in area seen in photo 2.
Vocal cord cancer (2 of 8)
Close-up view of only the anterior half of the cords. Notice the irregular surface, and areas of leukoplakia within this squamous cell carcinoma.
Vocal cord cancer (2 of 8)
Close-up view of only the anterior half of the cords. Notice the irregular surface, and areas of leukoplakia within this squamous cell carcinoma.
Vocal cord cancer, during radiation (3 of 8)
Just over midway through radiation treatment. One can see that the tumor is melting away.
Vocal cord cancer, during radiation (3 of 8)
Just over midway through radiation treatment. One can see that the tumor is melting away.
Vocal cord cancer, during radiation (4 of 8)
Postcricoid / hypopharyngeal mucositis. In this view, the patient is performing a so-called trumpet maneuver to splay open the lower throat. The radiation delivered to the vocal cords (which inhabit the airway but are hidden here due to the momentary constriction of the laryngeal vestibule, at arrows) also causes superficial ulceration of the swallowing passage (upper half of the photo), directly behind the vocal cords. On occasion, if tissue reaction and mucositis are much more severe than seen here, a stricture can form, requiring dilation.
Vocal cord cancer, during radiation (4 of 8)
Postcricoid / hypopharyngeal mucositis. In this view, the patient is performing a so-called trumpet maneuver to splay open the lower throat. The radiation delivered to the vocal cords (which inhabit the airway but are hidden here due to the momentary constriction of the laryngeal vestibule, at arrows) also causes superficial ulceration of the swallowing passage (upper half of the photo), directly behind the vocal cords. On occasion, if tissue reaction and mucositis are much more severe than seen here, a stricture can form, requiring dilation.
Vocal cord cancer, 2 months after radiation (5 of 8)
Two months after radiation is complete, showing that the tumor is gone, and the mucositis has resolved. There is a small anterior commissure web (at arrow) just below the free margin of the cords. The patient’s voice can nevertheless pass for normal.
Vocal cord cancer, 2 months after radiation (5 of 8)
Two months after radiation is complete, showing that the tumor is gone, and the mucositis has resolved. There is a small anterior commissure web (at arrow) just below the free margin of the cords. The patient’s voice can nevertheless pass for normal.
Vocal cord cancer, 4 months after radiation (6 of 8)
Now four months after the end of radiation. Close-up view of the postcricoid / hypopharynx regions (compare with photo 4 in this series). Mucositis here is resolved as well, and there is no stricture.
Vocal cord cancer, 4 months after radiation (6 of 8)
Now four months after the end of radiation. Close-up view of the postcricoid / hypopharynx regions (compare with photo 4 in this series). Mucositis here is resolved as well, and there is no stricture.
Vocal cord cancer, 6 months after radiation (7 of 8)
Now six months after the end of radiation. Strobe illumination, open phase of vibration. Note that the contours of the vocal cords are not perfectly normal, even though voice is very good.
Vocal cord cancer, 6 months after radiation (7 of 8)
Now six months after the end of radiation. Strobe illumination, open phase of vibration. Note that the contours of the vocal cords are not perfectly normal, even though voice is very good.
Vocal cord cancer, 6 months after radiation (8 of 8)
Strobe illumination, nearly closed phase of vibration. Oscillatory flexibility is preserved, but the vocal cord margins are not perfectly straight.
Vocal cord cancer, 6 months after radiation (8 of 8)
Strobe illumination, nearly closed phase of vibration. Oscillatory flexibility is preserved, but the vocal cord margins are not perfectly straight.
Vocal Cord Cancer, before and after Radiotherapy
Vocal cord cancer (1 of 7)
A 66-year-old man who complains of hoarseness. He smoked a pack a day for 50 years but quit five years ago. Note here the fullness and irregular contour especially of the left vocal cord ( right of photo). A biopsy confirmed this was cancer.
Vocal cord cancer (1 of 7)
A 66-year-old man who complains of hoarseness. He smoked a pack a day for 50 years but quit five years ago. Note here the fullness and irregular contour especially of the left vocal cord ( right of photo). A biopsy confirmed this was cancer.
Vocal cord cancer (2 of 7)
At closer range, scattered leukoplakia and stippled vascular markings (suggestive of HPV effect, but HPV tested negative).
Vocal cord cancer (2 of 7)
At closer range, scattered leukoplakia and stippled vascular markings (suggestive of HPV effect, but HPV tested negative).
Vocal cord cancer, 3 weeks after radiotherapy (4 of 7)
Same patient, three weeks after the end of full-course radiotherapy. Distant view shows radiation-induced mucositis on the false and true cords, seen as areas of white, superficial ulceration. General redness is also a radiation effect.
Vocal cord cancer, 3 weeks after radiotherapy (4 of 7)
Same patient, three weeks after the end of full-course radiotherapy. Distant view shows radiation-induced mucositis on the false and true cords, seen as areas of white, superficial ulceration. General redness is also a radiation effect.
Vocal cord cancer, 3 weeks after radiotherapy (5 of 7)
Closer view, showing that the main tumor of the left vocal cord (again, right of photo) has melted away. Note that the mucositis is generalized, and not necessarily focal to the area of tumor sloughing.
Vocal cord cancer, 3 weeks after radiotherapy (5 of 7)
Closer view, showing that the main tumor of the left vocal cord (again, right of photo) has melted away. Note that the mucositis is generalized, and not necessarily focal to the area of tumor sloughing.
Vocal cord cancer, 2 months after radiotherapy (6 of 7)
Same patient, now two months after the end of radiotherapy. All of the visible tumor is gone, and voice is very good. Small anterior web. Compare with photo 1 of this series.
Vocal cord cancer, 2 months after radiotherapy (6 of 7)
Same patient, now two months after the end of radiotherapy. All of the visible tumor is gone, and voice is very good. Small anterior web. Compare with photo 1 of this series.
Vocal cord cancer, 2 months after radiotherapy (7 of 7)
Phonation. Compare with photo 3 of this series.
Vocal cord cancer, 2 months after radiotherapy (7 of 7)
Phonation. Compare with photo 3 of this series.
Cancer, HPV Subtype 16, Before and After Radiation
Cancer: HPV Subtype 16 (1 of 5)
Cancer, in a patient with HPV subtype 16. The divot and blood seen on the left vocal cord (right of image) are the result of a biopsy performed elsewhere (not by BVI physician) earlier the same day as this examination.
Cancer: HPV Subtype 16 (1 of 5)
Cancer, in a patient with HPV subtype 16. The divot and blood seen on the left vocal cord (right of image) are the result of a biopsy performed elsewhere (not by BVI physician) earlier the same day as this examination.
Cancer: HPV Subtype 16, after radiation therapy (3 of 5)
Six weeks after the end of radiation therapy, the tumor is no longer seen. However, part of the left cord (right of image) is missing, due to sloughing of the tumor that had eaten away part of the cord’s normal tissue.
Cancer: HPV Subtype 16, after radiation therapy (3 of 5)
Six weeks after the end of radiation therapy, the tumor is no longer seen. However, part of the left cord (right of image) is missing, due to sloughing of the tumor that had eaten away part of the cord’s normal tissue.
Cancer: HPV Subtype 16, after radiation therapy (4 of 5)
Phonation. Strobe light, open phase of vibration, shows that the margin of the left cord (right of image) is at a lower level than the right’s, due to loss of some of the bulk of the cord where the tumor died and sloughed away.
Cancer: HPV Subtype 16, after radiation therapy (4 of 5)
Phonation. Strobe light, open phase of vibration, shows that the margin of the left cord (right of image) is at a lower level than the right’s, due to loss of some of the bulk of the cord where the tumor died and sloughed away.
Cancer: HPV Subtype 16, after radiation therapy (5 of 5)
Strobe light, closed phase of vibration. The more normal right cord (left of image) unsuccessfully attempts to reach the left cord’s residual upper surface mucosa. Voice is functional but hoarse.
Cancer: HPV Subtype 16, after radiation therapy (5 of 5)
Strobe light, closed phase of vibration. The more normal right cord (left of image) unsuccessfully attempts to reach the left cord’s residual upper surface mucosa. Voice is functional but hoarse.
Pre-and 1 Week Post-removal Vocal Nodules
Verrucous carcinoma (1 of 5)
Verrucous carcinoma, left vocal cord, persistent after radiotherapy elsewhere, in a patient unable to undergo general anesthesia due to severe lung disease.
Verrucous carcinoma (1 of 5)
Verrucous carcinoma, left vocal cord, persistent after radiotherapy elsewhere, in a patient unable to undergo general anesthesia due to severe lung disease.
During voicing (2 of 5)
Can be seen even during voicing.
During voicing (2 of 5)
Can be seen even during voicing.
After laser treatment (3 of 5)
After several Thulium Laser ablations, using topical and injected local anesthesia, with patient sitting in examination chair, thereby avoiding general anesthesia.
After laser treatment (3 of 5)
After several Thulium Laser ablations, using topical and injected local anesthesia, with patient sitting in examination chair, thereby avoiding general anesthesia.
Mucus remains several weeks after laser treatment (4 of 5)
Approximately six weeks later, durable resolution of tumor. Yellow material is mucus.
Mucus remains several weeks after laser treatment (4 of 5)
Approximately six weeks later, durable resolution of tumor. Yellow material is mucus.
Much improvement several weeks after laser treatment (5 of 5)
During voicing. Arytenoid moves, but much of membranous vocal cord has been ablated as intended.
Much improvement several weeks after laser treatment (5 of 5)
During voicing. Arytenoid moves, but much of membranous vocal cord has been ablated as intended.
For more info: Cancer
Capillary Ectasia:
Capillary Ectasia, before and after Laser Coagulation
Capillary ectasia (1 of 7)
Abducted, breathing position, standard light. This is a vascular abnormality and not a polyp. We use the term “capillary lake.”
Capillary ectasia (1 of 7)
Abducted, breathing position, standard light. This is a vascular abnormality and not a polyp. We use the term “capillary lake.”
Capillary ectasia (2 of 7)
Pre-phonatory instant, standard light.
Capillary ectasia (2 of 7)
Pre-phonatory instant, standard light.
Capillary ectasia (3 of 7)
Strobe light, open phase of vibration. Mucus is consistent with the patient’s known acid reflux laryngitis.
Capillary ectasia (3 of 7)
Strobe light, open phase of vibration. Mucus is consistent with the patient’s known acid reflux laryngitis.
Closed phase (4 of 7)
Strobe light, closed phase of vibration.
Closed phase (4 of 7)
Strobe light, closed phase of vibration.
Capillary ectasia, after laser coagulation (5 of 7)
Abducted breathing position, standard light, some weeks after pulsed-KTP laser coagulation of the dilated capillaries, which are no longer visible.
Capillary ectasia, after laser coagulation (5 of 7)
Abducted breathing position, standard light, some weeks after pulsed-KTP laser coagulation of the dilated capillaries, which are no longer visible.
Capillary ectasia, after laser coagulation (6 of 7)
Same view, but under narrow-band illumination.
Capillary ectasia, after laser coagulation (6 of 7)
Same view, but under narrow-band illumination.
After laser coagulation (7 of 7)
Prephonatory instant, narrow-band illumination.
After laser coagulation (7 of 7)
Prephonatory instant, narrow-band illumination.
Capillary ectasia (1 of 3)
Bilateral capillary ectasia, made to stand out with the help of narrow-band illumination.
Capillary ectasia (1 of 3)
Bilateral capillary ectasia, made to stand out with the help of narrow-band illumination.
Capillary ectasia, right after laser coagulation (2 of 3)
At the conclusion of pulsed-KTP laser coagulation, performed in a videoendoscopy procedure room with patient awake and sitting in a chair.
Capillary ectasia, right after laser coagulation (2 of 3)
At the conclusion of pulsed-KTP laser coagulation, performed in a videoendoscopy procedure room with patient awake and sitting in a chair.
Capillary ectasia, 6 weeks after laser coagulation (3 of 3)
Six weeks later; the capillaries have vanished, as expected.
Capillary ectasia, 6 weeks after laser coagulation (3 of 3)
Six weeks later; the capillaries have vanished, as expected.
Capillary Ectasia and Hemorrhagic Polyp, before and after Treatment
Capillary ectasia and hemorrhagic polyp (1 of 4)
Open phase of vibration, strobe light.
Capillary ectasia and hemorrhagic polyp (1 of 4)
Open phase of vibration, strobe light.
Capillary ectasia and hemorrhagic polyp (2 of 4)
Partially closed phase of vibration, strobe light.
Capillary ectasia and hemorrhagic polyp (2 of 4)
Partially closed phase of vibration, strobe light.
Capillary ectasia and hemorrhagic polyp, after treatment (3 of 4)
Abducted breathing position after vocal cord microsurgery, standard light. Note that the right cord is normalized, the capillary ectasia on the left is smaller, but persists in spite of spot-coagulation. A simple pulsed-KTP laser procedure in the videoendoscopy procedure room abolished this residual lesion.
Capillary ectasia and hemorrhagic polyp, after treatment (3 of 4)
Abducted breathing position after vocal cord microsurgery, standard light. Note that the right cord is normalized, the capillary ectasia on the left is smaller, but persists in spite of spot-coagulation. A simple pulsed-KTP laser procedure in the videoendoscopy procedure room abolished this residual lesion.
Capillary ectasia and hemorrhagic polyp, after treatment (4 of 4)
Pre-phonatory instant, standard light, showing excellent match. Oscillatory ability entirely normal into extreme upper range in this professional singer.
Capillary ectasia and hemorrhagic polyp, after treatment (4 of 4)
Pre-phonatory instant, standard light, showing excellent match. Oscillatory ability entirely normal into extreme upper range in this professional singer.
For more info: Capillary ectasia
RRP:
Papillomas, HPV Subtype 11, before and after Removal
Papillomas: HPV Subtype 11 (1 of 4)
Papillomas at posterior vocal cords, with left side (right of image) much larger than right. This patient has HPV subtype 11.
Papillomas: HPV Subtype 11 (1 of 4)
Papillomas at posterior vocal cords, with left side (right of image) much larger than right. This patient has HPV subtype 11.
Papillomas: HPV Subtype 11 (2 of 4)
Closer view, under narrow band illumination, which accentuates the vascular pattern.
Papillomas: HPV Subtype 11 (2 of 4)
Closer view, under narrow band illumination, which accentuates the vascular pattern.
Papillomas, removed: HPV Subtype 11 (3 of 4)
Two weeks after microsurgical removal, cidofovir injection, and return of normal voice.
Papillomas, removed: HPV Subtype 11 (3 of 4)
Two weeks after microsurgical removal, cidofovir injection, and return of normal voice.
Papillomas, removed: HPV Subtype 11 (4 of 4)
Closer view of left posterior vocal cord, narrow band illumination. Notice that there are a few dot-like vascular marks. These are typical of HPV effect, and may presage recurrence.
Papillomas, removed: HPV Subtype 11 (4 of 4)
Closer view of left posterior vocal cord, narrow band illumination. Notice that there are a few dot-like vascular marks. These are typical of HPV effect, and may presage recurrence.
Lesions and Papillomas of HPV, Before and After Removal and Adjuvant Injection
Lesions and papillomas of HPV subtype? (1 of 8)
At initial diagnosis, as yet untyped for HPV. Multi-focal lesions on both vocal cords.
Lesions and papillomas of HPV subtype? (1 of 8)
At initial diagnosis, as yet untyped for HPV. Multi-focal lesions on both vocal cords.
Subtle lesion (2 of 8)
Narrow-band illumination and a different viewing angle better reveal the more subtle lesion on the upper surface of the right cord (dotted circle).
Subtle lesion (2 of 8)
Narrow-band illumination and a different viewing angle better reveal the more subtle lesion on the upper surface of the right cord (dotted circle).
Open phase (3 of 8)
Strobe light, open phase of vibration, showing mismatch.
Open phase (3 of 8)
Strobe light, open phase of vibration, showing mismatch.
1 week after removal (4 of 8)
One week after removal of papillomas, voice is dramatically restored. Strobe light, open phase of vibration. Compare with photo 3.
1 week after removal (4 of 8)
One week after removal of papillomas, voice is dramatically restored. Strobe light, open phase of vibration. Compare with photo 3.
1 week after removal (5 of 8)
Strobe illumination, closed phase. Even in falsetto, oscillatory ability is preserved due to the precise and superficial removal of the papillomas.
1 week after removal (5 of 8)
Strobe illumination, closed phase. Even in falsetto, oscillatory ability is preserved due to the precise and superficial removal of the papillomas.
Injecting adjuvant (6 of 8)
At three weeks after removal, the patient regards his voice as normal. The patient has neither lesion nor vascular change to suggest any residual or recurrent lesion. Needle in photo (arrow) positioned to inject adjuvant medication in attempt to prevent recurrence. This procedure is done in a voice lab under topical anesthesia, not the operating room.
Injecting adjuvant (6 of 8)
At three weeks after removal, the patient regards his voice as normal. The patient has neither lesion nor vascular change to suggest any residual or recurrent lesion. Needle in photo (arrow) positioned to inject adjuvant medication in attempt to prevent recurrence. This procedure is done in a voice lab under topical anesthesia, not the operating room.
After injecting adjuvant (7 of 8)
After both cords have been “inflated” with adjuvant medication. Note the convex, slightly blanched vocal cord margins, due to superficial infiltration of the medication.
After injecting adjuvant (7 of 8)
After both cords have been “inflated” with adjuvant medication. Note the convex, slightly blanched vocal cord margins, due to superficial infiltration of the medication.
After final adjuvant injection (8 of 8)
Nearly a month later, immediately after the third and final adjuvant injection (hence the blood below the vocal cords). The patient again regarded his voice as completely normal. No sign at this early point of recurrence of papillomas or other HPV lesions. Patients with focal disease as seen in photo 1 of this series not infrequently go into long-term remission or “cure,” though it may be impossible to discern the relative roles of surgery, adjuvants, and the patient’s immune system.
After final adjuvant injection (8 of 8)
Nearly a month later, immediately after the third and final adjuvant injection (hence the blood below the vocal cords). The patient again regarded his voice as completely normal. No sign at this early point of recurrence of papillomas or other HPV lesions. Patients with focal disease as seen in photo 1 of this series not infrequently go into long-term remission or “cure,” though it may be impossible to discern the relative roles of surgery, adjuvants, and the patient’s immune system.
Papillomas, HPV Subtype 6, before and after Removal
Papillomas: HPV Subtype 6 (1 of 4)
Papilloma, left vocal cord (right of image), standard light. Voice is grossly hoarse. This patient has HPV subtype 6.
Papillomas: HPV Subtype 6 (1 of 4)
Papilloma, left vocal cord (right of image), standard light. Voice is grossly hoarse. This patient has HPV subtype 6.
Papillomas: HPV Subtype 6 (2 of 4)
Same lesion, under narrow band illumination.
Papillomas: HPV Subtype 6 (2 of 4)
Same lesion, under narrow band illumination.
Papillomas, removed: HPV Subtype 6 (3 of 4)
After removal and cidofovir injection, normalized larynx. Voice is normal.
Papillomas, removed: HPV Subtype 6 (3 of 4)
After removal and cidofovir injection, normalized larynx. Voice is normal.
Papillomas, removed: HPV Subtype 6 (4 of 4)
Same view, under narrow band illumination.
Papillomas, removed: HPV Subtype 6 (4 of 4)
Same view, under narrow band illumination.
For more info: Recurrent Respiratory Papillomatosis
Vocal Cord Paralysis/paresis:
TA-only Paresis before and after Voice Gel Injection
TA weakness, intact LCA + PCA (1 of 5)
TA weakness indicated by bowed margin and “spaghetti-linguini” difference between the cords. Medial turning of vocal process (arrow) suggests intact LCA; abducted position suggests intact PCA function. Blood is from cricothyroid membrane puncture to instill topical anesthesia.
TA weakness, intact LCA + PCA (1 of 5)
TA weakness indicated by bowed margin and “spaghetti-linguini” difference between the cords. Medial turning of vocal process (arrow) suggests intact LCA; abducted position suggests intact PCA function. Blood is from cricothyroid membrane puncture to instill topical anesthesia.
Prephonatory instant (2 of 5)
Before voice gel injection at prephonatory instant. Wasting of left cord (right of photo), and capacious ventricle on the left (right of photo) clearly evident.
Prephonatory instant (2 of 5)
Before voice gel injection at prephonatory instant. Wasting of left cord (right of photo), and capacious ventricle on the left (right of photo) clearly evident.
Gel injection (3 of 5)
At beginning of voice gel injection (needle at white arrow).
Gel injection (3 of 5)
At beginning of voice gel injection (needle at white arrow).
Straight vocal cord margin (4 of 5)
At conclusion of voice gel, note straight left cord margin (right of photo). Compare with photos 1 and 3.
Straight vocal cord margin (4 of 5)
At conclusion of voice gel, note straight left cord margin (right of photo). Compare with photos 1 and 3.
Phonation (5 of 5)
Phonation after injection complete. Voice dramatically strengthened. Compare with photo 2.
Phonation (5 of 5)
Phonation after injection complete. Voice dramatically strengthened. Compare with photo 2.
Paresis, TA-only: before and after an Implant
Paresis, TA-only (1 of 5)
During abducted breathing position, note the atrophy of the left cord (right of image), mild margin convexity, and the capacious ventricle (at bottom-right), all of which indicate TA paresis. The cord abducts fully, demonstrating intact PCA fuction. LCA function cannot be determined in this view.
Paresis, TA-only (1 of 5)
During abducted breathing position, note the atrophy of the left cord (right of image), mild margin convexity, and the capacious ventricle (at bottom-right), all of which indicate TA paresis. The cord abducts fully, demonstrating intact PCA fuction. LCA function cannot be determined in this view.
Paresis, TA-only (2 of 5)
Adducted position for phonation, with phonatory blurring as seen under standard light. LCA appears to be functioning, as indicated by the strict anterior-posterior direction of the left vocal process (right of image), just the same as for the right. This accounts for quite good approximation of the cords. The ventricle again appears capacious (dotted oval). Based upon these first two photos, we can surmise that this is a TA-only paresis.
Paresis, TA-only (2 of 5)
Adducted position for phonation, with phonatory blurring as seen under standard light. LCA appears to be functioning, as indicated by the strict anterior-posterior direction of the left vocal process (right of image), just the same as for the right. This accounts for quite good approximation of the cords. The ventricle again appears capacious (dotted oval). Based upon these first two photos, we can surmise that this is a TA-only paresis.
Paresis, TA-only (3 of 5)
Under strobe light, showing increased amplitude of vibration of the left cord (right of image). This finding suggests in yet another way that the TA muscle is paralyzed.
Paresis, TA-only (3 of 5)
Under strobe light, showing increased amplitude of vibration of the left cord (right of image). This finding suggests in yet another way that the TA muscle is paralyzed.
Paresis, TA-only: after implant is placed (4 of 5)
After placement of an implant into the left cord (right of image). Note the bulging of that cord and straightening of the cord's margin, and also that the ventricle on that side no longer appears capacious. Compare with photo 1.
Paresis, TA-only: after implant is placed (4 of 5)
After placement of an implant into the left cord (right of image). Note the bulging of that cord and straightening of the cord's margin, and also that the ventricle on that side no longer appears capacious. Compare with photo 1.
Paresis, TA-only: after implant is placed (5 of 5)
Under strobe illumination. Note that the lateral excursion of both cords is the same, since the left cord (right of image) is now less flaccid. Compare with photo 3
Paresis, TA-only: after implant is placed (5 of 5)
Under strobe illumination. Note that the lateral excursion of both cords is the same, since the left cord (right of image) is now less flaccid. Compare with photo 3
Vocal Cord Paralysis, Before and After Medialization
Vocal cord paralysis: before medialization (1 of 12)
A classic example of “spaghetti-linguine” vocal cords, here in breathing position. The “linguine” cord (left of image) is normal; the “spaghetti” cord (right of image) is paralyzed, likely since birth. On the paralyzed side, notice the deep and broad ventricle, mild bowing of the margin of the cord, and reduced width of the upper surface of the cord (“spaghetti”-like), as compared with the non-paralyzed side.
Vocal cord paralysis: before medialization (1 of 12)
A classic example of “spaghetti-linguine” vocal cords, here in breathing position. The “linguine” cord (left of image) is normal; the “spaghetti” cord (right of image) is paralyzed, likely since birth. On the paralyzed side, notice the deep and broad ventricle, mild bowing of the margin of the cord, and reduced width of the upper surface of the cord (“spaghetti”-like), as compared with the non-paralyzed side.
Vocal cord paralysis: before medialization (2 of 12)
Phonation, more distant view, under standard light. Notice the considerable gap between the vocal cords. This gap correlates with the patient’s weak and air-wasting voice quality.
Vocal cord paralysis: before medialization (2 of 12)
Phonation, more distant view, under standard light. Notice the considerable gap between the vocal cords. This gap correlates with the patient’s weak and air-wasting voice quality.
Increased amplitude + bowing (3 of 12)
Open phase of vibration, under strobe light. The paralyzed cord (right of image) has a much increased amplitude (lateral or outward excursion) and exaggerated bowing, due to its flaccidity.
Increased amplitude + bowing (3 of 12)
Open phase of vibration, under strobe light. The paralyzed cord (right of image) has a much increased amplitude (lateral or outward excursion) and exaggerated bowing, due to its flaccidity.
Paralyzed cord cannot close (4 of 12)
“Closed” phase of vibration, which is of course not closed at all, because the paralyzed cord (right of image) cannot come fully to the midline.
Paralyzed cord cannot close (4 of 12)
“Closed” phase of vibration, which is of course not closed at all, because the paralyzed cord (right of image) cannot come fully to the midline.
Visual symptoms are diminished (5 of 12)
One week after surgical medialization of the paralyzed cord (right of image), using a silastic implant buried deeply within the cord. Notice that the ventricle is no longer capacious, and the free margin is no longer bowed. Furthermore, in contrast with photo 1 of this series, the “spaghetti-linguine” description of these vocal cords is no longer apt.
Visual symptoms are diminished (5 of 12)
One week after surgical medialization of the paralyzed cord (right of image), using a silastic implant buried deeply within the cord. Notice that the ventricle is no longer capacious, and the free margin is no longer bowed. Furthermore, in contrast with photo 1 of this series, the “spaghetti-linguine” description of these vocal cords is no longer apt.
Gap is gone (6 of 12)
Phonation, under standard light. The gap between the cords is no longer seen (compare with photo 2), and the patient's spontaneous speaking voice sounds normal. She can recruit loudness effectively without any luffing or observable weakness.
Gap is gone (6 of 12)
Phonation, under standard light. The gap between the cords is no longer seen (compare with photo 2), and the patient's spontaneous speaking voice sounds normal. She can recruit loudness effectively without any luffing or observable weakness.
Similar lateral excursion (7 of 12)
Open phase of vibration, under strobe light. The lateral or outward excursion of the paralyzed cord (right of image) is now similar to that of the non-paralyzed cord. Compare with photo 3.
Similar lateral excursion (7 of 12)
Open phase of vibration, under strobe light. The lateral or outward excursion of the paralyzed cord (right of image) is now similar to that of the non-paralyzed cord. Compare with photo 3.
Closed phase is improved (8 of 12)
The closed phase of vibration is much more closed than preoperatively. Compare with photo 4.
Closed phase is improved (8 of 12)
The closed phase of vibration is much more closed than preoperatively. Compare with photo 4.
5 months after medialization (9 of 12)
Five months after medialization. Compare this partially abducted position with photos 1 and 5 of this series.
5 months after medialization (9 of 12)
Five months after medialization. Compare this partially abducted position with photos 1 and 5 of this series.
5 months after medialization (10 of 12)
Phonation, under standard light, showing vibratory blur. Compare with photos 2 and 6 of this series.
5 months after medialization (10 of 12)
Phonation, under standard light, showing vibratory blur. Compare with photos 2 and 6 of this series.
5 months after medialization (11 of 12)
Open phase of vibration, under strobe light. As in photo 7 of this series, and in contrast to photo 3, the implant does not permit the paralyzed cord (right of image) to “buckle” laterally, or outward. If anything, the vibratory excursion of the non-paralyzed (and un-implanted) cord is greater than that of the paralyzed, implanted cord.
5 months after medialization (11 of 12)
Open phase of vibration, under strobe light. As in photo 7 of this series, and in contrast to photo 3, the implant does not permit the paralyzed cord (right of image) to “buckle” laterally, or outward. If anything, the vibratory excursion of the non-paralyzed (and un-implanted) cord is greater than that of the paralyzed, implanted cord.
5 months after medialization (12 of 12)
The closed phase of vibration is now virtually normal, similar to photo 8 and in contrast with photo 4.
5 months after medialization (12 of 12)
The closed phase of vibration is now virtually normal, similar to photo 8 and in contrast with photo 4.
Paresis, TA + LCA, before and after Placement of an Implant
Paresis, TA + LCA (1 of 6)
Distant view shows lesser normal-appearing abduction left cord (right of image) during breathing, suggesting that the left posterior cricoarytenoid muscle is working. Note the lesser bulk of the left vocal cord as compared with the right, although this is subtle at this viewing distance.
Paresis, TA + LCA (1 of 6)
Distant view shows lesser normal-appearing abduction left cord (right of image) during breathing, suggesting that the left posterior cricoarytenoid muscle is working. Note the lesser bulk of the left vocal cord as compared with the right, although this is subtle at this viewing distance.
Paresis, TA + LCA (2 of 6)
At closer range, still in breathing position, one can see more easily the “linguine” of the right vocal cord (left of image) compared with the “spaghetti” and slight bowing of the left. These findings correlate with left thyroarytenoid (TA) muscle weakness and atrophy.
Paresis, TA + LCA (2 of 6)
At closer range, still in breathing position, one can see more easily the “linguine” of the right vocal cord (left of image) compared with the “spaghetti” and slight bowing of the left. These findings correlate with left thyroarytenoid (TA) muscle weakness and atrophy.
Paresis, TA + LCA (3 of 6)
In phonatory position under strobe light, the bowing of the left cord (right of image) is more evident, as is the lateral turning of the left vocal process, consistent with weakness of the left lateral cricoarytenoid (LCA) muscle. Lines denote the direction each vocal process is pointing.
Paresis, TA + LCA (3 of 6)
In phonatory position under strobe light, the bowing of the left cord (right of image) is more evident, as is the lateral turning of the left vocal process, consistent with weakness of the left lateral cricoarytenoid (LCA) muscle. Lines denote the direction each vocal process is pointing.
Paresis, TA + LCA: 1 week after implant is placed (4 of 6)
One week after placement of a large silastic implant into the left vocal cord (right of image). Notice the temporary eversion of the left ventricle, almost simulating a large polyp.
Paresis, TA + LCA: 1 week after implant is placed (4 of 6)
One week after placement of a large silastic implant into the left vocal cord (right of image). Notice the temporary eversion of the left ventricle, almost simulating a large polyp.
Paresis, TA + LCA: 3 months after implant is placed (5 of 6)
A few months later, fullness of left vocal cord (right of image) remains, but eversion / edema of ventricular mucosa has resolved. Compare with image 1.
Paresis, TA + LCA: 3 months after implant is placed (5 of 6)
A few months later, fullness of left vocal cord (right of image) remains, but eversion / edema of ventricular mucosa has resolved. Compare with image 1.
Paresis, TA + LCA: 3 months after implant is placed (6 of 6)
During phonation, much better closure (with markedly improved voice) but still slightly lateral turning of the left vocal process (right of image). Compare with image 3.
Paresis, TA + LCA: 3 months after implant is placed (6 of 6)
During phonation, much better closure (with markedly improved voice) but still slightly lateral turning of the left vocal process (right of image). Compare with image 3.
Paresis, TA + LCA, before and after Injection of Voice Gel
Paresis, TA + LCA (1 of 5)
Right vocal cord paresis (left of image). Note marked atrophy as compared with the left cord. Highly lateralized position denotes some persistent action of the right posterior cricoarytenoid muscle.
Paresis, TA + LCA (1 of 5)
Right vocal cord paresis (left of image). Note marked atrophy as compared with the left cord. Highly lateralized position denotes some persistent action of the right posterior cricoarytenoid muscle.
Paresis, TA + LCA (2 of 5)
Initiation of phonation. Note medical turning off left vocal process of arytenoid (right of image), and absent movement of the right vocal cord. Neither thyroarytenoid nor lateral cricoarytenoid muscles are innervated.
Paresis, TA + LCA (2 of 5)
Initiation of phonation. Note medical turning off left vocal process of arytenoid (right of image), and absent movement of the right vocal cord. Neither thyroarytenoid nor lateral cricoarytenoid muscles are innervated.
Voice gel injection (3 of 5)
Immediately following injection of right vocal cord (left of image) with voice gel, with patient in videoendoscopy room chair, under topical anesthesia. Note bulging of right vocal cord.
Voice gel injection (3 of 5)
Immediately following injection of right vocal cord (left of image) with voice gel, with patient in videoendoscopy room chair, under topical anesthesia. Note bulging of right vocal cord.
1 month after injection (4 of 5)
A month later, showing plumping up of the right vocal cord (left of image) with voice gel. Vocal cord continues to abduct fully, due to functioning posterior branch of recurrent nerve, which innervates the posterior cricoarytenoid muscle.
1 month after injection (4 of 5)
A month later, showing plumping up of the right vocal cord (left of image) with voice gel. Vocal cord continues to abduct fully, due to functioning posterior branch of recurrent nerve, which innervates the posterior cricoarytenoid muscle.
Phonation 1 month after injection (5 of 5)
Phonation. There is some movement to the midline due to the bilaterally innervated interarytenoid muscle. The lateral cricoarytenoid muscle is paralyzed, as seen in lateral turning of the vocal process. Voice is dramatically improved as compared with pre-injection. The voice gel will be expected to gradually absorb over three to nine months, during which time the anterior branch of the recurrent nerve may recover its function.
Phonation 1 month after injection (5 of 5)
Phonation. There is some movement to the midline due to the bilaterally innervated interarytenoid muscle. The lateral cricoarytenoid muscle is paralyzed, as seen in lateral turning of the vocal process. Voice is dramatically improved as compared with pre-injection. The voice gel will be expected to gradually absorb over three to nine months, during which time the anterior branch of the recurrent nerve may recover its function.
Voice Gel Injection for Vocal Cord Paralysis
Left vocal cord paralysis (1 of 4)
This photo shows left vocal cord paralysis in the breathing position. Note the margin bowing, “spaghetti-linguini” difference in bulk, and capacious ventricle. Note: the * is for comparison with photo 3.
Left vocal cord paralysis (1 of 4)
This photo shows left vocal cord paralysis in the breathing position. Note the margin bowing, “spaghetti-linguini” difference in bulk, and capacious ventricle. Note: the * is for comparison with photo 3.
Voice-making position (2 of 4)
Here the paralysis is shown in the voice-making position. Note the lateral buckling of the left vocal cord (right of photo). This flaccidity and the gap between the vocal cords explain the patient’s breathy (air-wasting) voice quality.
Voice-making position (2 of 4)
Here the paralysis is shown in the voice-making position. Note the lateral buckling of the left vocal cord (right of photo). This flaccidity and the gap between the vocal cords explain the patient’s breathy (air-wasting) voice quality.
Voice gel injection (3 of 4)
This is the same patient at the beginning of voice gel injection. Needle at arrow coming from subglottis upwards and laterally. At * one can see the beginning of bulging in the posterior ventricle. The vocal cord also looks slightly shifted towards the midline. Compare with photo 1.
Voice gel injection (3 of 4)
This is the same patient at the beginning of voice gel injection. Needle at arrow coming from subglottis upwards and laterally. At * one can see the beginning of bulging in the posterior ventricle. The vocal cord also looks slightly shifted towards the midline. Compare with photo 1.
After voice gel injection (4 of 4)
Voice-making position after voice gel injection is complete and bulge in ventricle at * is more evident. Closure is much better; the voice is dramatically stronger and the air-wasting quality much less. Compare with photo 2.
After voice gel injection (4 of 4)
Voice-making position after voice gel injection is complete and bulge in ventricle at * is more evident. Closure is much better; the voice is dramatically stronger and the air-wasting quality much less. Compare with photo 2.
For more info: Paralysis or Paresis
Stenosis:
Subglottic Stenosis, before and after Dilation
Subglottic stenosis (1 of 5)
Middle-aged woman with unexplained shortness of breath and noisy breathing, due to this idiopathic inflammatory and very high subglottic stenosis. The patient initially declined dilation due to her anxiety. She also had granularity of the nasal septum and a positive ANCA profile for Wegener’s granulomatosis.
Subglottic stenosis (1 of 5)
Middle-aged woman with unexplained shortness of breath and noisy breathing, due to this idiopathic inflammatory and very high subglottic stenosis. The patient initially declined dilation due to her anxiety. She also had granularity of the nasal septum and a positive ANCA profile for Wegener’s granulomatosis.
Subglottic stenosis, worsened (2 of 5)
Five months later, the symptoms became intolerable, and the stenosis was noted to be slightly narrower and with a greater posterior component. The patient agreed to dilation.
Subglottic stenosis, worsened (2 of 5)
Five months later, the symptoms became intolerable, and the stenosis was noted to be slightly narrower and with a greater posterior component. The patient agreed to dilation.
Subglottic stenosis, worsened (3 of 5)
Same exam as photo 2; this close-up view shows more clearly the inflammatory nature of this stenosis.
Subglottic stenosis, worsened (3 of 5)
Same exam as photo 2; this close-up view shows more clearly the inflammatory nature of this stenosis.
Subglottic stenosis, after dilation (4 of 5)
Five days after outpatient dilation, triamcinolone injection, and topical mitomycin C application. The patient’s symptoms have vanished, the harsh inspiratory noise is no longer heard, and the size of the airway, though still not normal, is more than doubled. Compare with photo 2 of this series.
Subglottic stenosis, after dilation (4 of 5)
Five days after outpatient dilation, triamcinolone injection, and topical mitomycin C application. The patient’s symptoms have vanished, the harsh inspiratory noise is no longer heard, and the size of the airway, though still not normal, is more than doubled. Compare with photo 2 of this series.
Subglottic stenosis, after dilation (5 of 5)
Same exam as photo 4, close-up view. Intensification of the inflammatory changes of this stenosis are expected so early after dilation. Compare size of the stenosis with photo 3 of this series.
Subglottic stenosis, after dilation (5 of 5)
Same exam as photo 4, close-up view. Intensification of the inflammatory changes of this stenosis are expected so early after dilation. Compare size of the stenosis with photo 3 of this series.
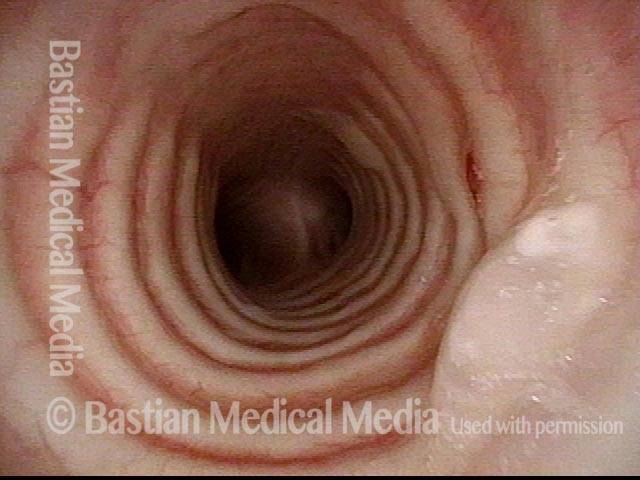
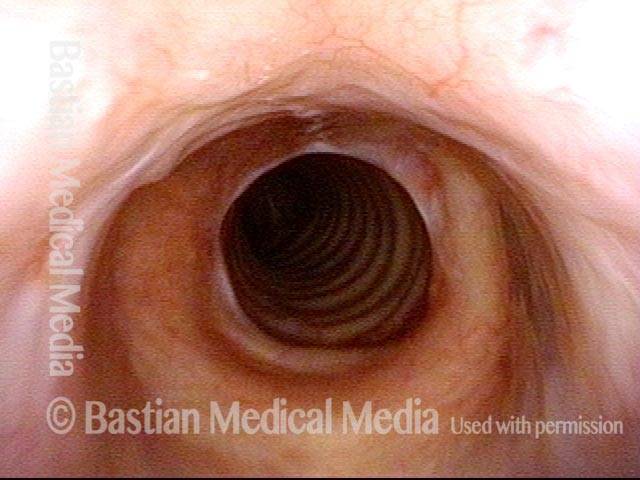
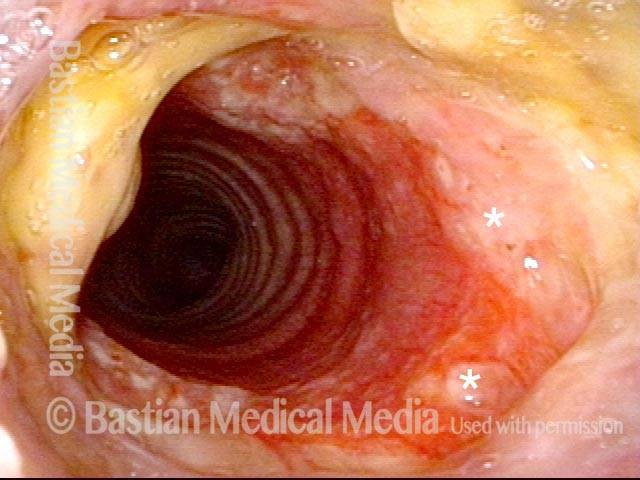
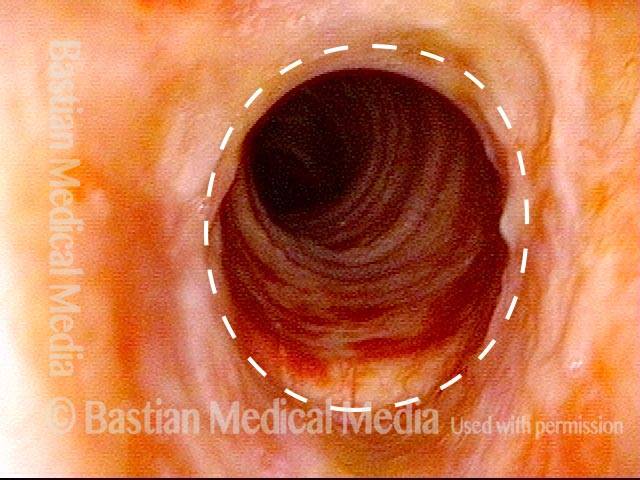
Subglottic stenosis, before dilation (1 of 2)
This individual has undergone at least a dozen prior dilations, each of which provides dramatic relief from noisy breathing and exercise intolerance. Here the patient is halfway to needing re-dilation, due to the typical inflammatory stenosis that is seen. Compare with photo 2.
Subglottic stenosis, before dilation (1 of 2)
This individual has undergone at least a dozen prior dilations, each of which provides dramatic relief from noisy breathing and exercise intolerance. Here the patient is halfway to needing re-dilation, due to the typical inflammatory stenosis that is seen. Compare with photo 2.
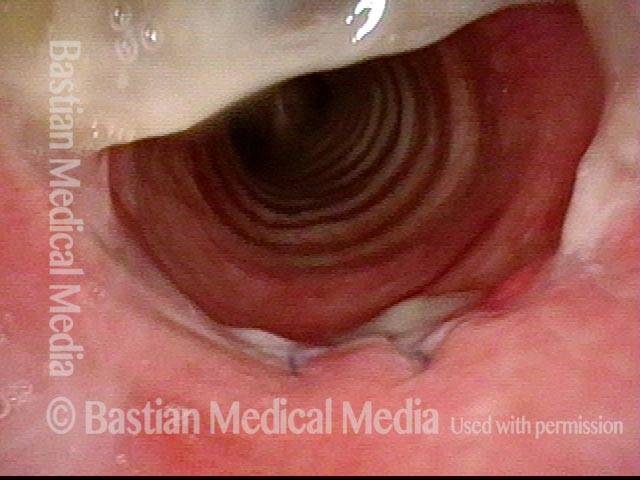
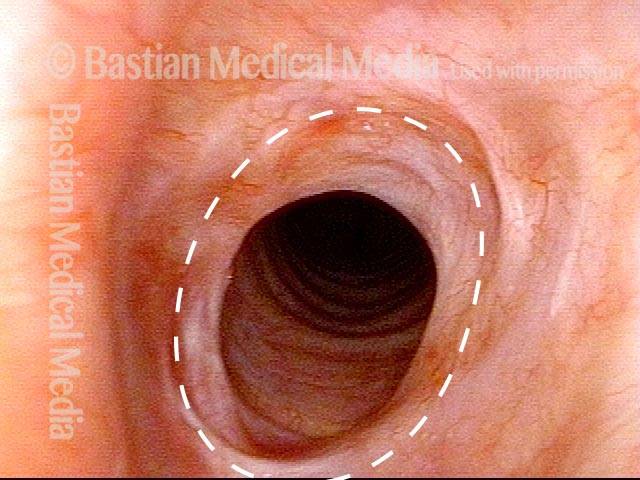
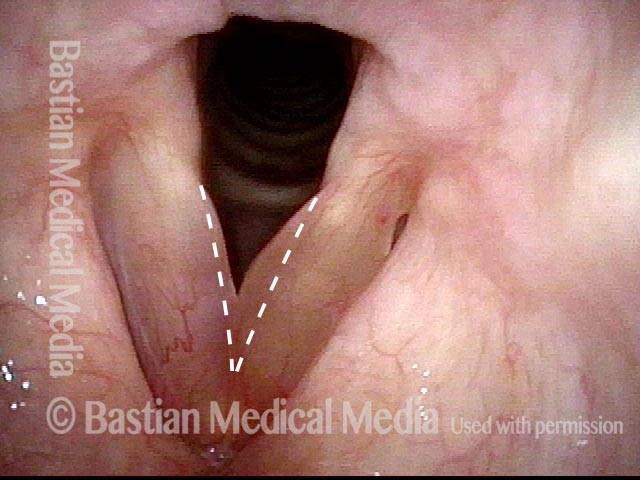
Subglottic stenosis, after dilation (2 of 2)
One week after one of this patient's dilations (with Kenalog injection and topical Mitomycin C), showing a dramatic widening of her airway; compare with photo 1. After a number of years, inflammatory lesions such as this sometimes "burn out," and the interval between dilations increases.
Subglottic stenosis, after dilation (2 of 2)
One week after one of this patient's dilations (with Kenalog injection and topical Mitomycin C), showing a dramatic widening of her airway; compare with photo 1. After a number of years, inflammatory lesions such as this sometimes "burn out," and the interval between dilations increases.
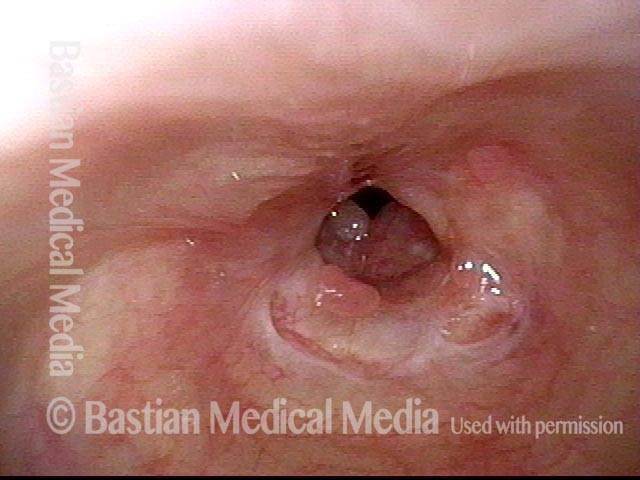
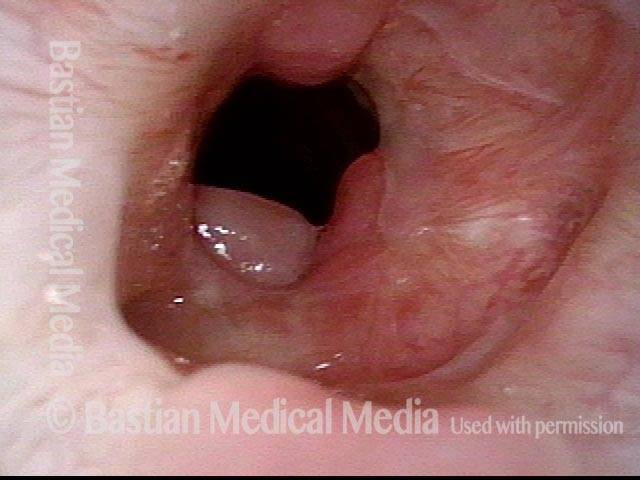
Tracheal Stenosis, before and after Tracheal Resection and Primary Reanastomosis
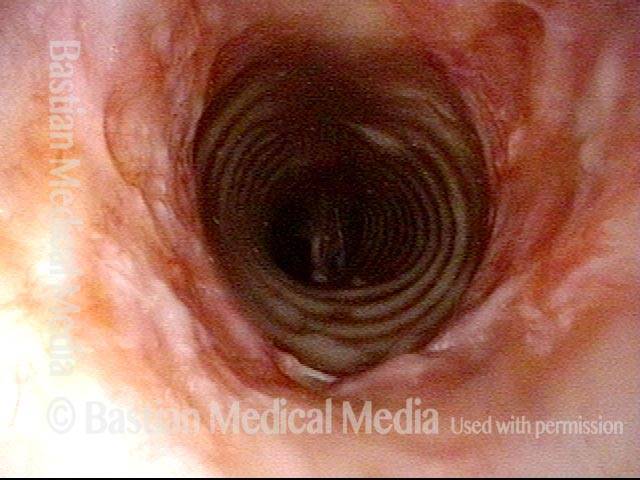
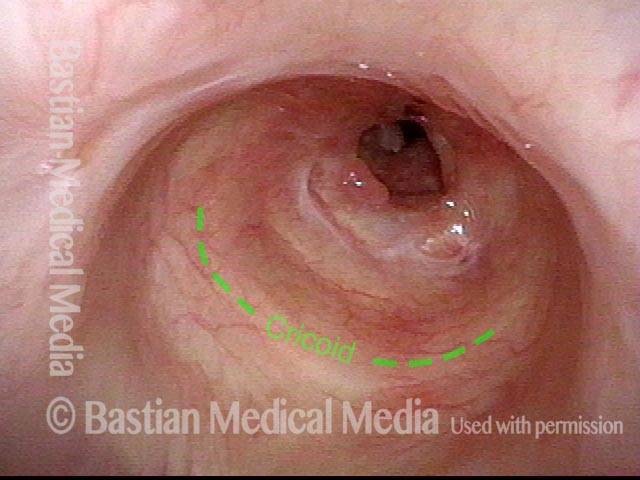
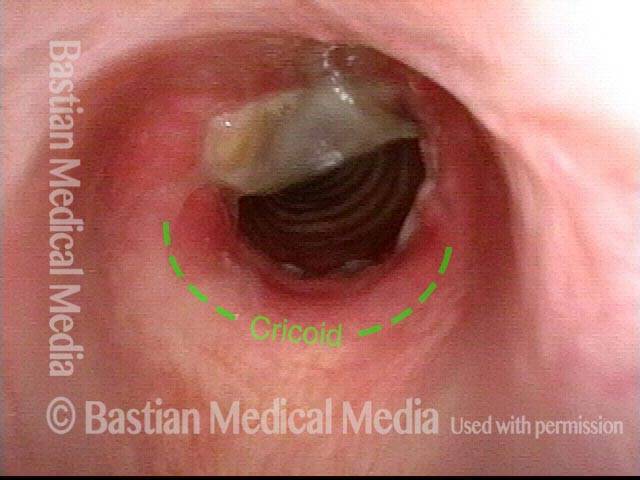
Tracheal stenosis (1 of 8)
This view from the level of the vocal cords shows high-grade tracheal stenosis, involving rings two, three, and four; the airway here is an estimated 30% of its normal diameter. For reference, a dotted line marks the level of the cricoid cartilage.
Tracheal stenosis (1 of 8)
This view from the level of the vocal cords shows high-grade tracheal stenosis, involving rings two, three, and four; the airway here is an estimated 30% of its normal diameter. For reference, a dotted line marks the level of the cricoid cartilage.
Tracheal stenosis (2 of 8)
Slightly closer view. This patient is very short of breath with minimal activity and, even at rest, has audible stridor. Elsewhere, across a span of several prior weeks, she had undergone three dilation procedures with only minimal, transient benefit.
Tracheal stenosis (2 of 8)
Slightly closer view. This patient is very short of breath with minimal activity and, even at rest, has audible stridor. Elsewhere, across a span of several prior weeks, she had undergone three dilation procedures with only minimal, transient benefit.
Close up view (3 of 8)
Close-up view shows scarring, collapse of tracheal walls, and some granulation tissue.
Close up view (3 of 8)
Close-up view shows scarring, collapse of tracheal walls, and some granulation tissue.
Just below the tracheal stenosis (4 of 8)
The trachea just beyond the stenosis is normal.
Just below the tracheal stenosis (4 of 8)
The trachea just beyond the stenosis is normal.
Tracheal stenosis, 5 days after surgery (5 of 8)
Five days after tracheal resection and primary reanastomosis. Compare with photo 1. Note that the airway’s diameter has at least tripled (part of the opening is obscured by tenacious mucus). The patient’s shortness of breath is now gone, as is the stridor.
Tracheal stenosis, 5 days after surgery (5 of 8)
Five days after tracheal resection and primary reanastomosis. Compare with photo 1. Note that the airway’s diameter has at least tripled (part of the opening is obscured by tenacious mucus). The patient’s shortness of breath is now gone, as is the stridor.
Tracheal stenosis, 5 days after surgery (6 of 8)
Close-up of the line of anastomosis, with a couple of sutures visible. Compare with photo 3. Again, tenacious mucus in the upper part of the photo is obscuring part of the view.
Tracheal stenosis, 5 days after surgery (6 of 8)
Close-up of the line of anastomosis, with a couple of sutures visible. Compare with photo 3. Again, tenacious mucus in the upper part of the photo is obscuring part of the view.
Tracheal stenosis, 2 months after surgery (7 of 8)
Another eight weeks later. The airway is wide-open and has also now healed since the surgery. Compare this view with photo 1 (pre-surgery) and photo 5 (early follow-up).
Tracheal stenosis, 2 months after surgery (7 of 8)
Another eight weeks later. The airway is wide-open and has also now healed since the surgery. Compare this view with photo 1 (pre-surgery) and photo 5 (early follow-up).
Tracheal stenosis, 2 months after surgery (8 of 8)
Compare this view with photo 2.
Tracheal stenosis, 2 months after surgery (8 of 8)
Compare this view with photo 2.
Tracheal Deformity and Stenosis, before and after Repair
Upper tracheal stenosis, before repair (1 of 6)
View from above the level of the vocal cords, showing severe narrowing and deformity of the upper trachea, caused by a “difficult” tracheotomy many years earlier. This man is frustrated by activity limitations, and difficulty coughing up accumulated mucus.
Upper tracheal stenosis, before repair (1 of 6)
View from above the level of the vocal cords, showing severe narrowing and deformity of the upper trachea, caused by a “difficult” tracheotomy many years earlier. This man is frustrated by activity limitations, and difficulty coughing up accumulated mucus.
Upper tracheal stenosis, before repair (2 of 6)
View from just below the level of the vocal cords, showing the deformity and stenosis more clearly.
Upper tracheal stenosis, before repair (2 of 6)
View from just below the level of the vocal cords, showing the deformity and stenosis more clearly.
Looking from below the stenosis (3 of 6)
The trachea below the area of stenosis is normal.
Looking from below the stenosis (3 of 6)
The trachea below the area of stenosis is normal.
After tracheal repair (4 of 6)
View from just above the level of the vocal cords, six weeks after tracheal resection and primary anastomosis, showing final result. Patient feels he breathes completely normally. Compare with photo 1.
After tracheal repair (4 of 6)
View from just above the level of the vocal cords, six weeks after tracheal resection and primary anastomosis, showing final result. Patient feels he breathes completely normally. Compare with photo 1.
After tracheal repair (5 of 6)
View from just below the level of the vocal cords. The tracheal caliber is now virtually normal. A broken, absorbable suture is seen at 2 o’clock, and a tiny remaining unhealed area is at 11 o'clock. Compare with photo 2.
After tracheal repair (5 of 6)
View from just below the level of the vocal cords. The tracheal caliber is now virtually normal. A broken, absorbable suture is seen at 2 o’clock, and a tiny remaining unhealed area is at 11 o'clock. Compare with photo 2.
After tracheal repair (6 of 6)
A close-up view shows the circular line of the anastomosis more clearly.
After tracheal repair (6 of 6)
A close-up view shows the circular line of the anastomosis more clearly.
For more info: Stenosis
Other:
Candida Laryngitis, before and after Treatment
Candida laryngitis (1 of 4)
Severe laryngeal candidiasis, in a person using inhaled steroids at high dose. Standard light.
Candida laryngitis (1 of 4)
Severe laryngeal candidiasis, in a person using inhaled steroids at high dose. Standard light.
Candida laryngitis (2 of 4)
Closer view shows more clearly not only the white areas, but also surrounding inflammation. Standard light.
Candida laryngitis (2 of 4)
Closer view shows more clearly not only the white areas, but also surrounding inflammation. Standard light.
Candida laryngitis, 15 days after starting treatment (3 of 4)
After 15 days of oral fluconazole. Obvious improvement, but incomplete resolution of tissue changes.
Candida laryngitis, 15 days after starting treatment (3 of 4)
After 15 days of oral fluconazole. Obvious improvement, but incomplete resolution of tissue changes.
Candida laryngitis, several months later (4 of 4)
After longer-term fluconazole, along with reduction of inhaled steroid dose, complete resolution. Strobe light, closed phase of vibration at high vocal pitch.
Candida laryngitis, several months later (4 of 4)
After longer-term fluconazole, along with reduction of inhaled steroid dose, complete resolution. Strobe light, closed phase of vibration at high vocal pitch.
Epidermoid Cyst, before and after Removal
Epidermoid cyst (1 of 3)
Epidermoid cyst, right vocal cord. Note white submucosal mass predominately on upper surface of the cord, but with bilateral free margin elevation as well.
Epidermoid cyst (1 of 3)
Epidermoid cyst, right vocal cord. Note white submucosal mass predominately on upper surface of the cord, but with bilateral free margin elevation as well.
Epidermoid cyst (2 of 3)
Close view, initiation of phonation under strobe light.
Epidermoid cyst (2 of 3)
Close view, initiation of phonation under strobe light.
Epidermoid cyst, removed (3 of 3)
After submucosal dissection and removal of cyst. Free margin swelling remains, as one cannot straighten the free margin (remove redundant mucosa stretched over the cyst) at the same time as cyst removal.
Epidermoid cyst, removed (3 of 3)
After submucosal dissection and removal of cyst. Free margin swelling remains, as one cannot straighten the free margin (remove redundant mucosa stretched over the cyst) at the same time as cyst removal.
Choral Singer’s Voice Fully Restored
Hoarse choral singer (1 of 4)
Older amateur but experienced and committed choral singer is grossly hoarse due to this chronic vascular lesion, left vocal cord (right of photo).
Hoarse choral singer (1 of 4)
Older amateur but experienced and committed choral singer is grossly hoarse due to this chronic vascular lesion, left vocal cord (right of photo).
Residual bruising (2 of 4)
Less than a week after surgical removal, residual bruising is seen.
Residual bruising (2 of 4)
Less than a week after surgical removal, residual bruising is seen.
2 months post-surgery (3 of 4)
Nearly 2 months after surgery, only a bit of ectasia (non-threatening as it is upper surface e of the cord) is seen. The patient says voice is fully restored.
2 months post-surgery (3 of 4)
Nearly 2 months after surgery, only a bit of ectasia (non-threatening as it is upper surface e of the cord) is seen. The patient says voice is fully restored.
Margins match (4 of 4)
Under strobe light, the margins match perfectly at D5 (587 Hz) and vibratory flexibility is equal to the unoperated side.
Margins match (4 of 4)
Under strobe light, the margins match perfectly at D5 (587 Hz) and vibratory flexibility is equal to the unoperated side.
Medialization, before and after
Before medialization (1 of 4)
Right vocal cord (left of photo) paralysis. During breathing as shown here, the right cord (left of photo) is paramedian, while the left cord (right of photo) is widely abducted. Note also the bowing (curvature) of the right cord (left of photo), and the prominent ventricle, both of which are indications of atrophy/ reduced bulk of the muscle within that cord.
Before medialization (1 of 4)
Right vocal cord (left of photo) paralysis. During breathing as shown here, the right cord (left of photo) is paramedian, while the left cord (right of photo) is widely abducted. Note also the bowing (curvature) of the right cord (left of photo), and the prominent ventricle, both of which are indications of atrophy/ reduced bulk of the muscle within that cord.
Before medialization (2 of 4)
Phonation, with vibratory blurring under standard light. Right cord (left of photo) remains paramedian, and the left cord (right of photo) has swung to the midline. Even so, large gap between the cords shows that closure is incomplete, and this explains the weak, air-wasting voice; the patient can only say a few words before running out of air and having to take another breath.
Before medialization (2 of 4)
Phonation, with vibratory blurring under standard light. Right cord (left of photo) remains paramedian, and the left cord (right of photo) has swung to the midline. Even so, large gap between the cords shows that closure is incomplete, and this explains the weak, air-wasting voice; the patient can only say a few words before running out of air and having to take another breath.
After medialization (3 of 4)
After medialization and placement of a silastic implant, the right cord (left of photo) is less atrophied and the bowing much diminished. The cord has also been shifted slightly to the midline.
After medialization (3 of 4)
After medialization and placement of a silastic implant, the right cord (left of photo) is less atrophied and the bowing much diminished. The cord has also been shifted slightly to the midline.
After medialization (4 of 4)
Phonation, again with vibratory blurring, but notice the much better approximation of the cords. The voice is stronger, and less air-wasting.
After medialization (4 of 4)
Phonation, again with vibratory blurring, but notice the much better approximation of the cords. The voice is stronger, and less air-wasting.
Airway Stenosis Caused By Wegener’s Granulomatosis, Before and After Dilations
Airway stenosis (1 of 5)
Marked inflammatory narrowing in the immediate subglottis. Within the ring of arrows is the inflamed, reddened tissue, which is narrowing the airway into the shape of a slit. This man needs to be active for his work, but notices shortness of breath and noisy breathing with exertion.
Airway stenosis (1 of 5)
Marked inflammatory narrowing in the immediate subglottis. Within the ring of arrows is the inflamed, reddened tissue, which is narrowing the airway into the shape of a slit. This man needs to be active for his work, but notices shortness of breath and noisy breathing with exertion.
Airway stenosis, after dilation (2 of 5)
Nine days after a dilation procedure, with local steroid injection and painting with Mitomycin C. The airway has widened, so that it is more oval-shaped and less slit-like. Compare with photo 1. Although a degree of stenosis remains, symptoms have subsided dramatically. For reference, asterisks mark the same points in the subglottis in this photo and the next photo.
Airway stenosis, after dilation (2 of 5)
Nine days after a dilation procedure, with local steroid injection and painting with Mitomycin C. The airway has widened, so that it is more oval-shaped and less slit-like. Compare with photo 1. Although a degree of stenosis remains, symptoms have subsided dramatically. For reference, asterisks mark the same points in the subglottis in this photo and the next photo.
Airway stenosis, after dilation (3 of 5)
Same exam, looking beyond the immediate subglottis. There is an inflammatory response that involves several centimeters of the upper trachea. Inflammatory areas often “trap” mucus, as seen here.
Airway stenosis, after dilation (3 of 5)
Same exam, looking beyond the immediate subglottis. There is an inflammatory response that involves several centimeters of the upper trachea. Inflammatory areas often “trap” mucus, as seen here.
Airway stenosis, before another dilation (4 of 5)
Now five months after the dilation procedure mentioned in photos 2 and 3. The patient has been receiving systemic treatment with methotrexate and prednisone. General appearance of the inflammation has decreased. In spite of this, as expected, the stenosis has persisted (dotted oval shows the estimated caliber or width of a normal airway) and symptoms have gradually increased. Thus, another dilation was scheduled for the next day.
Airway stenosis, before another dilation (4 of 5)
Now five months after the dilation procedure mentioned in photos 2 and 3. The patient has been receiving systemic treatment with methotrexate and prednisone. General appearance of the inflammation has decreased. In spite of this, as expected, the stenosis has persisted (dotted oval shows the estimated caliber or width of a normal airway) and symptoms have gradually increased. Thus, another dilation was scheduled for the next day.
Airway stenosis, after another dilation (5 of 5)
A week after photo 4, following the most recent dilation. There is expected immediate postoperative inflammation and an increase in the airway’s caliber or width by an estimated 30% (dotted oval again shows the estimated caliber or width of a normal airway; compare with photo 4). Symptoms are again abolished.
Airway stenosis, after another dilation (5 of 5)
A week after photo 4, following the most recent dilation. There is expected immediate postoperative inflammation and an increase in the airway’s caliber or width by an estimated 30% (dotted oval again shows the estimated caliber or width of a normal airway; compare with photo 4). Symptoms are again abolished.
Smoker’s Polyps, before and after Surgery
Smoker's polyps, BEFORE surgery (1 of 4)
Even during quiet breathing, the convexity of the vocal cord margins (dotted lines show where normal margins would be) reveal the presence of smoker's polyps.
Smoker's polyps, BEFORE surgery (1 of 4)
Even during quiet breathing, the convexity of the vocal cord margins (dotted lines show where normal margins would be) reveal the presence of smoker's polyps.
Smoker's polyps, BEFORE surgery (2 of 4)
During inspiratory phonation: the polyps are drawn inward and are easier to see.
Smoker's polyps, BEFORE surgery (2 of 4)
During inspiratory phonation: the polyps are drawn inward and are easier to see.
Smoker's polyps, AFTER surgery (3 of 4)
Two months after surgery, during quiet breathing. The vocal cord margins are now straight.
Smoker's polyps, AFTER surgery (3 of 4)
Two months after surgery, during quiet breathing. The vocal cord margins are now straight.
Smoker's polyps, AFTER surgery (4 of 4)
During inspiratory phonation: the margins are drawn into a mildly convex contour, but far less than preoperatively. The patient's voice is also much improved, albeit the occasional syllable dropouts due to recentness of surgery (listen to this patient's voice samples in the audio section of the encyclopedia entry).
Smoker's polyps, AFTER surgery (4 of 4)
During inspiratory phonation: the margins are drawn into a mildly convex contour, but far less than preoperatively. The patient's voice is also much improved, albeit the occasional syllable dropouts due to recentness of surgery (listen to this patient's voice samples in the audio section of the encyclopedia entry).
Leukoplakia, Before, During, and After Laser Coagulation
Leukoplakia, not yet seen (1 of 6)
A few years earlier, this patient underwent superficial laser cordectomy of the right vocal cord (left of photo) for cancer. The voice result is excellent, and the patient is being seen this day for a routine interval examination, and has no new complaints.
Leukoplakia, not yet seen (1 of 6)
A few years earlier, this patient underwent superficial laser cordectomy of the right vocal cord (left of photo) for cancer. The voice result is excellent, and the patient is being seen this day for a routine interval examination, and has no new complaints.
Leukoplakia (2 of 6)
At closer range, tiny points of leukoplakia (inside the green dotted oval) become evident. The bright white spot in the photo is just a light reflection.
Leukoplakia (2 of 6)
At closer range, tiny points of leukoplakia (inside the green dotted oval) become evident. The bright white spot in the photo is just a light reflection.
Leukoplakia (3 of 6)
Still closer view, again confirming the tiny patches of leukoplakia. There is another light reflection in this view, right in the middle of the photo.
Leukoplakia (3 of 6)
Still closer view, again confirming the tiny patches of leukoplakia. There is another light reflection in this view, right in the middle of the photo.
Leukoplakia, coagulated by laser (4 of 6)
Thulium laser coagulation of the leukoplakia lesions, through a glass fiber (blue-ish cylinder at top-right of photo), as seen under narrow-band illumination. The Thulium laser had been placed on stand-by prior to the routine examination, to save the patient a potential second visit. The coagulated tissue is also white, but will slough off within a few days, and along with it, the leukoplakia.
Leukoplakia, coagulated by laser (4 of 6)
Thulium laser coagulation of the leukoplakia lesions, through a glass fiber (blue-ish cylinder at top-right of photo), as seen under narrow-band illumination. The Thulium laser had been placed on stand-by prior to the routine examination, to save the patient a potential second visit. The coagulated tissue is also white, but will slough off within a few days, and along with it, the leukoplakia.
Leukoplakia, 3 months after laser treatment (5 of 6)
Three months after laser treatment, the patient has healed.
Leukoplakia, 3 months after laser treatment (5 of 6)
Three months after laser treatment, the patient has healed.
Leukoplakia, 3 months after laser treatment (6 of 6)
Three months after laser treatment, a close up view shows no signs of leukoplakia spots.
Leukoplakia, 3 months after laser treatment (6 of 6)
Three months after laser treatment, a close up view shows no signs of leukoplakia spots.
Anterior Saccular Cyst, before and after Removal
Anterior saccular cyst (1 of 4)
Phonation, open phase of vibration, under strobe light. Left-sided cyst (right of image) causes mildly rough voice quality.
Anterior saccular cyst (1 of 4)
Phonation, open phase of vibration, under strobe light. Left-sided cyst (right of image) causes mildly rough voice quality.
Anterior saccular cyst (2 of 4)
Four years later. Phonation, open phase of vibration, under strobe light. The cyst has enlarged, and voice quality has deteriorated. The patient wants this removed.
Anterior saccular cyst (2 of 4)
Four years later. Phonation, open phase of vibration, under strobe light. The cyst has enlarged, and voice quality has deteriorated. The patient wants this removed.
Anterior saccular cyst, removed (3 of 4)
Ten days after laser dissection of the complete cyst (not simple unroofing). At close range, looking into the left ventricle. The raw area (at arrows) is the bed of excision.
Anterior saccular cyst, removed (3 of 4)
Ten days after laser dissection of the complete cyst (not simple unroofing). At close range, looking into the left ventricle. The raw area (at arrows) is the bed of excision.
Anterior saccular cyst, removed (4 of 4)
Phonation, standard light. Some residual bruising of the left vocal cord (right of image), but voice quality and capabilities are normal.
Anterior saccular cyst, removed (4 of 4)
Phonation, standard light. Some residual bruising of the left vocal cord (right of image), but voice quality and capabilities are normal.
Leukoplakia, Before and After Surgical Removal
Diffuse leukoplakia (1 of 7)
Diffuse leukoplakia (seen under standard light) in a man who had undergone removal elsewhere at least twice, with rapid return of diffuse disease on both vocal cords.
Diffuse leukoplakia (1 of 7)
Diffuse leukoplakia (seen under standard light) in a man who had undergone removal elsewhere at least twice, with rapid return of diffuse disease on both vocal cords.
HPV effect (2 of 7)
Closer view, using narrow-band illumination. Leukoplakia is accentuated, but punctate vascular markings are also accentuated. We sometimes call this “HPV effect,” though in fact this man’s HPV subtyping was negative.
HPV effect (2 of 7)
Closer view, using narrow-band illumination. Leukoplakia is accentuated, but punctate vascular markings are also accentuated. We sometimes call this “HPV effect,” though in fact this man’s HPV subtyping was negative.
Surgical view (3 of 7)
Initial operative view. Note inversion of the photo (the “V” of lab view has become ^ in the operative view.
Surgical view (3 of 7)
Initial operative view. Note inversion of the photo (the “V” of lab view has become ^ in the operative view.
Basement membrane peeling (4 of 7)
During “basement membrane peeling.” One can see that the leukoplakia has been peeled like a piece of parchment. There is more bleeding than is often seen with this technique, but vibratory function should not be damaged because there has been no entry of Reinke’s space (lamina propria).
Basement membrane peeling (4 of 7)
During “basement membrane peeling.” One can see that the leukoplakia has been peeled like a piece of parchment. There is more bleeding than is often seen with this technique, but vibratory function should not be damaged because there has been no entry of Reinke’s space (lamina propria).
Removal complete (5 of 7)
After complete removal of the leukoplakia on not only right, but also left vocal fold.
Removal complete (5 of 7)
After complete removal of the leukoplakia on not only right, but also left vocal fold.
Two years post-op (6 of 7)
Two years after one superficial yet intensely precise peeling of the leukoplakia, plus one follow-up thulium laser ablation of scattered residual disease. The patient, a tenor, considers his voice to be normal. Closed phase of vibration, as seen under strobe light.
Two years post-op (6 of 7)
Two years after one superficial yet intensely precise peeling of the leukoplakia, plus one follow-up thulium laser ablation of scattered residual disease. The patient, a tenor, considers his voice to be normal. Closed phase of vibration, as seen under strobe light.
Mucosa is flexible (7 of 7)
Open phase of vibration, demonstrating that the mucosa on both vocal cords remains flexible. The shifting hazy patches seen here and in photo 6 are collections of mucus.
Mucosa is flexible (7 of 7)
Open phase of vibration, demonstrating that the mucosa on both vocal cords remains flexible. The shifting hazy patches seen here and in photo 6 are collections of mucus.
Redundant Supraglottic Mucosa, before and after Surgery
Redundant supraglottic mucosa (1 of 8)
This patient said that, when she breathes in (inspires), she hears noise and feels a sense of obstruction, especially if she inspires rapidly or with her chin down. She had found it necessary to use CPAP while reading with chin down, for example. Here, the patient is breathing quietly, with chin in neutral position, “looking at the horizon.” The vocal cords are abducted, the laryngeal vestibule is open, and there is no breathing noise.
Redundant supraglottic mucosa (1 of 8)
This patient said that, when she breathes in (inspires), she hears noise and feels a sense of obstruction, especially if she inspires rapidly or with her chin down. She had found it necessary to use CPAP while reading with chin down, for example. Here, the patient is breathing quietly, with chin in neutral position, “looking at the horizon.” The vocal cords are abducted, the laryngeal vestibule is open, and there is no breathing noise.
Supraglottic tissue (2 of 8)
Upon request, with her head position unchanged, the patient begins to inspire rapidly. As she inspires, supraglottic tissue (of the arytenoids and aryepiglottic cords) is drawn into the laryngeal vestibule, and harsh breathing noise is heard. This indrawn tissue, which is redundant mucosa, partially obscures the view of the still-abducted vocal cords (dotted lines).
Supraglottic tissue (2 of 8)
Upon request, with her head position unchanged, the patient begins to inspire rapidly. As she inspires, supraglottic tissue (of the arytenoids and aryepiglottic cords) is drawn into the laryngeal vestibule, and harsh breathing noise is heard. This indrawn tissue, which is redundant mucosa, partially obscures the view of the still-abducted vocal cords (dotted lines).
Vibrating mucosal margin (3 of 8)
At the peak of rapid inspiration. The vocal cords are still fully abducted (dotted lines) but almost completely obscured by the indrawn mucosa. In addition to the rushing sound, there is also a low-pitched fluttering or rumbling sound, caused by vibration of the leading edge of the indrawn supraglottic mucosa. “B” marks this blurred, vibrating mucosal margin.
Vibrating mucosal margin (3 of 8)
At the peak of rapid inspiration. The vocal cords are still fully abducted (dotted lines) but almost completely obscured by the indrawn mucosa. In addition to the rushing sound, there is also a low-pitched fluttering or rumbling sound, caused by vibration of the leading edge of the indrawn supraglottic mucosa. “B” marks this blurred, vibrating mucosal margin.
1 week after surgery (4 of 8)
Same patient, one week after laser peeling of the redundant mucosa. The areas which were addressed include: the upper face of the arytenoid, apical mucosa, aryepiglottic mucosa adjacent to the arytenoid, mucosa from the postarytenoid surface, and even some postcricoid mucosa.
1 week after surgery (4 of 8)
Same patient, one week after laser peeling of the redundant mucosa. The areas which were addressed include: the upper face of the arytenoid, apical mucosa, aryepiglottic mucosa adjacent to the arytenoid, mucosa from the postarytenoid surface, and even some postcricoid mucosa.
1 week after surgery (5 of 8)
At the peak of rapid inspiration. The aryepiglottic cords are drawn in slightly, but the laryngeal vestibule remains widely open. Compare with photo 3.
1 week after surgery (5 of 8)
At the peak of rapid inspiration. The aryepiglottic cords are drawn in slightly, but the laryngeal vestibule remains widely open. Compare with photo 3.
1 week after surgery (6 of 8)
Closer view of surgical details, showing that the entire posterior surface of the arytenoids has been denuded. An asterisk marks the edema at the cut edge of the postcricoid mucosa.
1 week after surgery (6 of 8)
Closer view of surgical details, showing that the entire posterior surface of the arytenoids has been denuded. An asterisk marks the edema at the cut edge of the postcricoid mucosa.
7 weeks after surgery (7 of 8)
Same patient, now seven weeks since laser peeling of the redundant mucosa. As the patient rapidly inspires here, the laryngeal vestibule remains widely open. The patient says that she no longer feels a sense of obstruction or hears noise when breathing, even with her chin down. Compare with photos 2 and 3.
7 weeks after surgery (7 of 8)
Same patient, now seven weeks since laser peeling of the redundant mucosa. As the patient rapidly inspires here, the laryngeal vestibule remains widely open. The patient says that she no longer feels a sense of obstruction or hears noise when breathing, even with her chin down. Compare with photos 2 and 3.
7 weeks after surgery (8 of 8)
The areas that were operated on have healed. Compare with photo 6.
7 weeks after surgery (8 of 8)
The areas that were operated on have healed. Compare with photo 6.
Cricopharyngeal Dysfunction, before and after Myotomy
Cricopharyngeal dysfunction: before myotomy (1 of 2)
Lateral x-ray of the neck while swallowing barium (the dark material seen here in the throat). The non-relaxing cricopharyngeus muscle (light-grey bulge outlined by a dotted line) is causing narrowing of the upper esophageal passageway, as highlighted by the narrowed stream of dark barium at that point (arrow). Liquids and very soft foods can squeak through this narrow opening, but solid foods tend to get stuck.
Cricopharyngeal dysfunction: before myotomy (1 of 2)
Lateral x-ray of the neck while swallowing barium (the dark material seen here in the throat). The non-relaxing cricopharyngeus muscle (light-grey bulge outlined by a dotted line) is causing narrowing of the upper esophageal passageway, as highlighted by the narrowed stream of dark barium at that point (arrow). Liquids and very soft foods can squeak through this narrow opening, but solid foods tend to get stuck.
Cricopharyngeal dysfunction: after myotomy, resolved (1 of 2)
After myotomy. The surgically divided muscle can no longer narrow the upper esophageal passageway, as seen by the widened stream of dark barium at the level of the muscle (arrows).
Cricopharyngeal dysfunction: after myotomy, resolved (1 of 2)
After myotomy. The surgically divided muscle can no longer narrow the upper esophageal passageway, as seen by the widened stream of dark barium at the level of the muscle (arrows).
Cricopharyngeal dysfunction: before myotomy (1 of 2)
Elderly patient with nearly a year’s duration of frequent lodgment of solid food at the level of the cricoid cartilage (at the mid-neck level). Note here the cricopharyngeus muscle “bar” which narrows the barium stream (indicated by green dotted line). This narrowing is due to incomplete relaxation of the muscle (aka upper esophageal sphincter) causing a smaller entrance to the esophagus. Liquids and very soft foods can still get through, but solid foods tend to get stuck or to require repeated swallows.
Cricopharyngeal dysfunction: before myotomy (1 of 2)
Elderly patient with nearly a year’s duration of frequent lodgment of solid food at the level of the cricoid cartilage (at the mid-neck level). Note here the cricopharyngeus muscle “bar” which narrows the barium stream (indicated by green dotted line). This narrowing is due to incomplete relaxation of the muscle (aka upper esophageal sphincter) causing a smaller entrance to the esophagus. Liquids and very soft foods can still get through, but solid foods tend to get stuck or to require repeated swallows.
Cricopharyngeal dysfunction: after myotomy (2 of 2)
A month after endoscopic (through the mouth) cricopharyngeus myotomy (division of the muscle with a laser). The patient’s initial swallowing symptoms are completely resolved and the barium stream no longer shows narrowing and the cricopharyngeus bar is no longer seen (see green arrows).
Cricopharyngeal dysfunction: after myotomy (2 of 2)
A month after endoscopic (through the mouth) cricopharyngeus myotomy (division of the muscle with a laser). The patient’s initial swallowing symptoms are completely resolved and the barium stream no longer shows narrowing and the cricopharyngeus bar is no longer seen (see green arrows).
Bilateral Laryngocele, before and after Removal
Bilateral laryngocele (1 of 8)
Vocal cords approaching point of best closure possible (due to left cord paresis). Faint dotted lines outline the approximate boundary of each laryngeal saccule, which not yet inflated.
Bilateral laryngocele (1 of 8)
Vocal cords approaching point of best closure possible (due to left cord paresis). Faint dotted lines outline the approximate boundary of each laryngeal saccule, which not yet inflated.
Bilateral laryngocele (2 of 8)
As air just begins coming upward between the cords, one can see subtle inflation (dotted lines), particularly of the right saccule (left of image).
Bilateral laryngocele (2 of 8)
As air just begins coming upward between the cords, one can see subtle inflation (dotted lines), particularly of the right saccule (left of image).
Bilateral laryngocele (3 of 8)
As phonation continues, inflation of the (now diagnosable) laryngocele becomes obvious, and the left laryngocele (right of image) is now more obviously inflated than before, again indicated by the dotted lines.
Bilateral laryngocele (3 of 8)
As phonation continues, inflation of the (now diagnosable) laryngocele becomes obvious, and the left laryngocele (right of image) is now more obviously inflated than before, again indicated by the dotted lines.
Bilateral laryngocele (4 of 8)
Near the end of a sustained period of voicing, maximum inflation of the laryngoceles is seen (dotted lines). On the right side (left of image), the stretching mucosa is so thinned as to appear translucent.
Bilateral laryngocele (4 of 8)
Near the end of a sustained period of voicing, maximum inflation of the laryngoceles is seen (dotted lines). On the right side (left of image), the stretching mucosa is so thinned as to appear translucent.
Bilateral laryngocele, after removal (5 of 8)
Same patient, breathing position, 12 weeks after complete removal of the bilateral laryngoceles via false cord incisions (lines of incision shown by dotted lines). This patient also has long-standing paralysis of the right vocal cord (left of image) and limited mobility of the left cord, so the cords don’t open fully for breathing.
Bilateral laryngocele, after removal (5 of 8)
Same patient, breathing position, 12 weeks after complete removal of the bilateral laryngoceles via false cord incisions (lines of incision shown by dotted lines). This patient also has long-standing paralysis of the right vocal cord (left of image) and limited mobility of the left cord, so the cords don’t open fully for breathing.
Bilateral laryngocele, after removal (6 of 8)
Phonatory position. Note the lack of inflation of the now-absent laryngoceles, and compare that with photos 3 and 4 of this series.
Bilateral laryngocele, after removal (6 of 8)
Phonatory position. Note the lack of inflation of the now-absent laryngoceles, and compare that with photos 3 and 4 of this series.
Bilateral laryngocele, after removal (7 of 8)
Closer view of the posterior ends of the true vocal cords during maximal abduction for breathing. Space between the vocal cords is an estimated 50% of normal, because of the paralyzed right cord and the limited mobility of the left cord.
Bilateral laryngocele, after removal (7 of 8)
Closer view of the posterior ends of the true vocal cords during maximal abduction for breathing. Space between the vocal cords is an estimated 50% of normal, because of the paralyzed right cord and the limited mobility of the left cord.
Bilateral laryngocele, after removal (8 of 8)
Same close-up view, but during phonation. The left vocal cord (right of image) has shifted slightly toward the midline, but the cords do not actually close and, thus, the patient cannot produce glottic (true vocal cord) voice. An implant could help to close this gap, but the patient will first try developing a “false cord voice.”
Bilateral laryngocele, after removal (8 of 8)
Same close-up view, but during phonation. The left vocal cord (right of image) has shifted slightly toward the midline, but the cords do not actually close and, thus, the patient cannot produce glottic (true vocal cord) voice. An implant could help to close this gap, but the patient will first try developing a “false cord voice.”
Vocal Cord Synechia: before, during, and after Surgery
Vocal Cord Synechia (1 of 9)
Post-intubation synechia tethers the arytenoid cartilages together. This patient is tracheotomy-dependent.
Vocal Cord Synechia (1 of 9)
Post-intubation synechia tethers the arytenoid cartilages together. This patient is tracheotomy-dependent.
Vocal cord synechia, during surgery (2 of 9)
Operative view of synechia ("v" of the vocal cords is inverted). Notice that the vocal cords are completely approximated because the synechia has bound them together.
Vocal cord synechia, during surgery (2 of 9)
Operative view of synechia ("v" of the vocal cords is inverted). Notice that the vocal cords are completely approximated because the synechia has bound them together.
Vocal cord synechia, during surgery (3 of 9)
Tiny forceps is separating the cords (arrows) and more clearly shows the extent of the synechia.
Vocal cord synechia, during surgery (3 of 9)
Tiny forceps is separating the cords (arrows) and more clearly shows the extent of the synechia.
Vocal cord synechia, during surgery (4 of 9)
Micro-scissors in position to divide the synechia cleanly. For perspective, the blade of the scissors is only a few millimeters long.
Vocal cord synechia, during surgery (4 of 9)
Micro-scissors in position to divide the synechia cleanly. For perspective, the blade of the scissors is only a few millimeters long.
Vocal cord synechia, during surgery (5 of 9)
After division of the synechia and topical application of an anti-scarring agent.
Vocal cord synechia, during surgery (5 of 9)
After division of the synechia and topical application of an anti-scarring agent.
Vocal cord synechia, after surgery (6 of 9)
Five days after surgery. Vocal cords are able to separate for breathing, and the tracheotomy tube can be removed. Compare with photo 1.
Vocal cord synechia, after surgery (6 of 9)
Five days after surgery. Vocal cords are able to separate for breathing, and the tracheotomy tube can be removed. Compare with photo 1.
Vocal Cord Synechia, after surgery (7 of 9)
Completely healed larynx after release of synechia. Abduction completely restored.
Vocal Cord Synechia, after surgery (7 of 9)
Completely healed larynx after release of synechia. Abduction completely restored.
Vocal cord synechia, after surgery (8 of 9)
As the vocal cords are coming together for phonation (not yet completely adducted).
Vocal cord synechia, after surgery (8 of 9)
As the vocal cords are coming together for phonation (not yet completely adducted).
Vocal cord synechia, after surgery (9 of 9)
Closer view. Can hardly see where the synechia was. Compare again with photo 1.
Vocal cord synechia, after surgery (9 of 9)
Closer view. Can hardly see where the synechia was. Compare again with photo 1.
Amyloidosis, before and after Debulking
Amyloidosis, before debulking (1 of 4)
Panoramic view. Submucosal amyloid deposits in pharyngeal walls and epiglottis, with examples at arrows.
Amyloidosis, before debulking (1 of 4)
Panoramic view. Submucosal amyloid deposits in pharyngeal walls and epiglottis, with examples at arrows.
Amyloidosis, before debulking (2 of 4)
View of anterior subglottis shows diffuse infiltration of yellowish, submucosal amyloid.
Amyloidosis, before debulking (2 of 4)
View of anterior subglottis shows diffuse infiltration of yellowish, submucosal amyloid.
Amyloidosis, before debulking (3 of 4)
Phonation under strobe light shows deposit at anterior commissure tethering upper surface of left vocal cord, and resultant vibratory asymmetry.
Amyloidosis, before debulking (3 of 4)
Phonation under strobe light shows deposit at anterior commissure tethering upper surface of left vocal cord, and resultant vibratory asymmetry.
Amyloidosis, after debulking (4 of 4)
Some months later, after laser debulking at short arrow, showing improvement of vibratory closure after tethering reduced. Note increase of nearby supraglottic amyloid deposit does not need to be addressed because it has no functional consequence to the patient, who has other asymptomatic deposits in subglottis, epiglottis, and nasopharynx.
Amyloidosis, after debulking (4 of 4)
Some months later, after laser debulking at short arrow, showing improvement of vibratory closure after tethering reduced. Note increase of nearby supraglottic amyloid deposit does not need to be addressed because it has no functional consequence to the patient, who has other asymptomatic deposits in subglottis, epiglottis, and nasopharynx.
Amyloidosis, before debulking (1 of 8)
Panoramic view of a large spherical mass of amyloid material bulging submucosally (contour at dotted line) in the false and aryepiglottic cords. The patient has only a harsh stage whisper, and glottic voice only with inspiratory (inhaling) phonation. The posterior vocal cord and ventricle of the opposite side (left of photo) are visible, but the amyloid mass obscures all but a few millimeters of the posterior cord on its side.
Amyloidosis, before debulking (1 of 8)
Panoramic view of a large spherical mass of amyloid material bulging submucosally (contour at dotted line) in the false and aryepiglottic cords. The patient has only a harsh stage whisper, and glottic voice only with inspiratory (inhaling) phonation. The posterior vocal cord and ventricle of the opposite side (left of photo) are visible, but the amyloid mass obscures all but a few millimeters of the posterior cord on its side.
Amyloidosis, before debulking (2 of 8)
Closer view.
Amyloidosis, before debulking (2 of 8)
Closer view.
Amyloidosis, before debulking (3 of 8)
View yet further down, showing the upper trachea (top-center of photo), the posterior end of one of the vocal cords (left of photo), and the edge of the amyloid mass (bottom-right of photo, again marked by a dotted line). The mass is again obscuring a view of the vocal cord on its side; in fact, it is pressing the cord downward, which affects the voice.
Amyloidosis, before debulking (3 of 8)
View yet further down, showing the upper trachea (top-center of photo), the posterior end of one of the vocal cords (left of photo), and the edge of the amyloid mass (bottom-right of photo, again marked by a dotted line). The mass is again obscuring a view of the vocal cord on its side; in fact, it is pressing the cord downward, which affects the voice.
Amyloidosis, before debulking (4 of 8)
Phonation, while attempting to see the vocal cords beyond the large overhanging mass. The vocal cords are each marked with an F, one of the posterior ventricles with a V, and the amyloid mass with an A. This mass actually overlies most of both vocal cords and presses them both downward.
Amyloidosis, before debulking (4 of 8)
Phonation, while attempting to see the vocal cords beyond the large overhanging mass. The vocal cords are each marked with an F, one of the posterior ventricles with a V, and the amyloid mass with an A. This mass actually overlies most of both vocal cords and presses them both downward.
Amyloidosis, after debulking (5 of 8)
Twenty-four hours after laser removal, i.e., debulking.
Amyloidosis, after debulking (5 of 8)
Twenty-four hours after laser removal, i.e., debulking.
Amyloidosis, after debulking (6 of 8)
Looking into the deepest recess of the dissection. The parallel lines indicate the upper border of the thyroid cartilage.
Amyloidosis, after debulking (6 of 8)
Looking into the deepest recess of the dissection. The parallel lines indicate the upper border of the thyroid cartilage.
Amyloidosis, after debulking (7 of 8)
After debulking, both vocal cords are now visible from this angle (compare with photo 2). Remaining swollen overhanging tissue did not appear to be infiltrated with amyloid deposition.
Amyloidosis, after debulking (7 of 8)
After debulking, both vocal cords are now visible from this angle (compare with photo 2). Remaining swollen overhanging tissue did not appear to be infiltrated with amyloid deposition.
Complete healing (8 of 8)
Many months after debulking, and with complete healing, the voice can pass for normal, and the laryngeal vestibule is no longer filled with the enormous bulk of amyloid. The nubbin at anterior false cord would only need removal if it became large enough to interfere with voice.
Complete healing (8 of 8)
Many months after debulking, and with complete healing, the voice can pass for normal, and the laryngeal vestibule is no longer filled with the enormous bulk of amyloid. The nubbin at anterior false cord would only need removal if it became large enough to interfere with voice.
Teflon Bulge, before and after Removal
Teflon bulge (1 of 4)
Abducted, breathing position, standard light. The left vocal cord (right of image) was injected with Teflon paste decades ago, before contemporary materials and techniques were available. Note the bulge in the ventricle, and also at the free margin of the cord (arrows).
Teflon bulge (1 of 4)
Abducted, breathing position, standard light. The left vocal cord (right of image) was injected with Teflon paste decades ago, before contemporary materials and techniques were available. Note the bulge in the ventricle, and also at the free margin of the cord (arrows).
Teflon bulge (2 of 4)
Phonatory view, strobe light. Notice how the right vocal cord (left of image) must “wrap around” the convex left vocal cord.
Teflon bulge (2 of 4)
Phonatory view, strobe light. Notice how the right vocal cord (left of image) must “wrap around” the convex left vocal cord.
Teflon bulge: after removal (3 of 4)
A few weeks after microsurgical “excavation” of part of the Teflon. Straighter free margin, and reduced bulge within the ventricle.
Teflon bulge: after removal (3 of 4)
A few weeks after microsurgical “excavation” of part of the Teflon. Straighter free margin, and reduced bulge within the ventricle.
Teflon bulge: after removal (4 of 4)
Phonation, strobe light. In spite of blurring, can see that the match of the cords is improved, and this correlates with the patient’s much improved voice.
Teflon bulge: after removal (4 of 4)
Phonation, strobe light. In spite of blurring, can see that the match of the cords is improved, and this correlates with the patient’s much improved voice.
Arytenoid Chondritis, before and after Removal
Arytenoid chondritis (1 of 5)
Festering arytenoid chondritis of over a year's duration. Several biopsies done elsewhere showed only inflammation.
Arytenoid chondritis (1 of 5)
Festering arytenoid chondritis of over a year's duration. Several biopsies done elsewhere showed only inflammation.
Arytenoid chondritis, removed (2 of 5)
Two weeks after aggressive partial arytenoid superstructure excision, in an attempt to get down to healthy cartilage.
Arytenoid chondritis, removed (2 of 5)
Two weeks after aggressive partial arytenoid superstructure excision, in an attempt to get down to healthy cartilage.
Arytenoid chondritis, removed and healed (3 of 5)
After complete healing. Note loss of anterior arytenoid prominence on the operated side as compared with the unoperated side.
Arytenoid chondritis, removed and healed (3 of 5)
After complete healing. Note loss of anterior arytenoid prominence on the operated side as compared with the unoperated side.
Arytenoid chondritis, removed and healed (4 of 5)
At this point, patient is entirely symptom-free. Notice resolution of the lesion and inflammation. The arytenoid mound is a little lower on right (left of image) than on left (right of image), due to surgical removal of part of the superstructure of the arytenoid.
Arytenoid chondritis, removed and healed (4 of 5)
At this point, patient is entirely symptom-free. Notice resolution of the lesion and inflammation. The arytenoid mound is a little lower on right (left of image) than on left (right of image), due to surgical removal of part of the superstructure of the arytenoid.
Arytenoid chondritis, removed and healed (5 of 5)
The area of festering chondritis has completely healed. The arrow shows center of where the lesion was.
Arytenoid chondritis, removed and healed (5 of 5)
The area of festering chondritis has completely healed. The arrow shows center of where the lesion was.
Congenital Glottic Sulcus and Bowing, before and after Injection
Glottic sulcus (1 of 10)
This young patient has a husky, air-wasting voice quality. View of the vocal cords, in breathing position. An abnormality can be seen, especially on the right cord (left of photo, at arrows).
Glottic sulcus (1 of 10)
This young patient has a husky, air-wasting voice quality. View of the vocal cords, in breathing position. An abnormality can be seen, especially on the right cord (left of photo, at arrows).
Glottic sulcus (2 of 10)
Under strobe lighting, during phonation, open phase of vibration, at a normal speech frequency (pitch), showing an unusually large amplitude of vibration.
Glottic sulcus (2 of 10)
Under strobe lighting, during phonation, open phase of vibration, at a normal speech frequency (pitch), showing an unusually large amplitude of vibration.
Glottic sulcus (3 of 10)
Closed phase of vibration, but not quite closing completely.
Glottic sulcus (3 of 10)
Closed phase of vibration, but not quite closing completely.
Glottic sulcus (4 of 10)
Closer view, during inspiratory phonation, reveals very clearly that this patient has sulci on both cords, with the open pocket especially visible on the right cord (left of photo).
Glottic sulcus (4 of 10)
Closer view, during inspiratory phonation, reveals very clearly that this patient has sulci on both cords, with the open pocket especially visible on the right cord (left of photo).
Sulcus with bowing, just prior to injection (5 of 10)
At the prephonatory instant, under standard light. In addition to a sulcus, this patient has congenital bowing.
Sulcus with bowing, just prior to injection (5 of 10)
At the prephonatory instant, under standard light. In addition to a sulcus, this patient has congenital bowing.
Sulcus with bowing, just prior to injection (6 of 10)
Phonation, under standard light, at the pitch E-flat 4 (~311 Hz). Notice in particular the generous width of the zone of vibratory blurring, which correlates with the flaccid, large-amplitude vibration seen in photo 2's strobe view.
Sulcus with bowing, just prior to injection (6 of 10)
Phonation, under standard light, at the pitch E-flat 4 (~311 Hz). Notice in particular the generous width of the zone of vibratory blurring, which correlates with the flaccid, large-amplitude vibration seen in photo 2's strobe view.
Voice gel injection (7 of 10)
The left vocal cord (right of photo) is now being injected with voice gel. The injection is centered so that the undersurface, free margin, and ventricle all show evidence of bulging.
Voice gel injection (7 of 10)
The left vocal cord (right of photo) is now being injected with voice gel. The injection is centered so that the undersurface, free margin, and ventricle all show evidence of bulging.
Voice gel injection (8 of 10)
The other vocal cord is now being injected.
Voice gel injection (8 of 10)
The other vocal cord is now being injected.
After the injection (9 of 10)
After voice gel injection is completed. At the prephonatory instant. Notice the reduced gap between the vocal cords (compare with photo 5).
After the injection (9 of 10)
After voice gel injection is completed. At the prephonatory instant. Notice the reduced gap between the vocal cords (compare with photo 5).
After the injection (10 of 10)
During phonation, under standard light, again at E-flat 4 (~311 Hz). The width of vibratory blurring is reduced (compare with photo 6), consistent with reduced amplitude of vibration and reduced air-wasting.
After the injection (10 of 10)
During phonation, under standard light, again at E-flat 4 (~311 Hz). The width of vibratory blurring is reduced (compare with photo 6), consistent with reduced amplitude of vibration and reduced air-wasting.
Glottic Sulcus, before and after Surgery
Glottic sulcus, before surgery (1 of 3)
Glottic sulcus, normal light, showing retained material/ granulation emerging from within the sulcus. There is a partial ring of capillaries around the sulcus on the right (left of photo), but no significant vessels within the sulcus (also see next photo).
Glottic sulcus, before surgery (1 of 3)
Glottic sulcus, normal light, showing retained material/ granulation emerging from within the sulcus. There is a partial ring of capillaries around the sulcus on the right (left of photo), but no significant vessels within the sulcus (also see next photo).
Glottic sulcus, before surgery (2 of 3)
Same patient. Narrow-band illumination shows the vascular markings more clearly.
Glottic sulcus, before surgery (2 of 3)
Same patient. Narrow-band illumination shows the vascular markings more clearly.
Glottic sulcus, after surgery (3 of 3)
Same patient, after surgery. Note microvasculature where it was not present prior to operation; especially noticeable on the right side (left of photo). There is now a continuous layer of mucosa.
Glottic sulcus, after surgery (3 of 3)
Same patient, after surgery. Note microvasculature where it was not present prior to operation; especially noticeable on the right side (left of photo). There is now a continuous layer of mucosa.
The Evolution of a Cricopharyngeus Myotomy Wound
Difficulty swallowing solid foods (1 of 8)
This ~80 year old man is having considerable trouble swallowing, particularly for solid foods. In this panoramic view at the start of VESS, saliva is noted in the swallowing crescent (outlined) and clinging to the posterior pharyngeal wall (arrows).
Difficulty swallowing solid foods (1 of 8)
This ~80 year old man is having considerable trouble swallowing, particularly for solid foods. In this panoramic view at the start of VESS, saliva is noted in the swallowing crescent (outlined) and clinging to the posterior pharyngeal wall (arrows).
Pooled saliva (2 of 8)
At closer range, the pooled saliva in the swallowing crescent is more clearly seen, as is some saliva within the laryngeal vestibule (arrows). Organized pooling of saliva or food / liquid can indicate cricopharyngeus dysfunction (non-relaxation).
Pooled saliva (2 of 8)
At closer range, the pooled saliva in the swallowing crescent is more clearly seen, as is some saliva within the laryngeal vestibule (arrows). Organized pooling of saliva or food / liquid can indicate cricopharyngeus dysfunction (non-relaxation).
Muscle bulge (3 of 8)
The patient has swallowed some water to clear away the saliva, and the pre-myotomy cricopharyngeus muscle bulge (between dotted lines) is seen with only a slit of opening into the esophagus at the arrow.
Muscle bulge (3 of 8)
The patient has swallowed some water to clear away the saliva, and the pre-myotomy cricopharyngeus muscle bulge (between dotted lines) is seen with only a slit of opening into the esophagus at the arrow.
Residue in swallow crescent (4 of 8)
After many boluses of blue-stained applesauce, the swallowing crescent remains full of residue, but laryngeal vestibule is not soiled. Both propulsive and receptive functions of swallowing are impaired but a significant part is outlet obstruction caused by incomplete cricopharyngeus muscle relaxation.
Residue in swallow crescent (4 of 8)
After many boluses of blue-stained applesauce, the swallowing crescent remains full of residue, but laryngeal vestibule is not soiled. Both propulsive and receptive functions of swallowing are impaired but a significant part is outlet obstruction caused by incomplete cricopharyngeus muscle relaxation.
Three weeks later (5 of 8)
About 3 weeks after cricopharyngeus myotomy, note that the salivary pooling in the swallowing "crescent" is less than pre-operation.
Three weeks later (5 of 8)
About 3 weeks after cricopharyngeus myotomy, note that the salivary pooling in the swallowing "crescent" is less than pre-operation.
Residual "wound" (6 of 8)
After administering blue-stained applesauce and water, the residual "wound" from the myotomy is stained blue. After myotomy, the cut ends of the muscle retracts laterally as suggested by the curved lines. Compare with the muscle bulge in Photo 3.
Residual "wound" (6 of 8)
After administering blue-stained applesauce and water, the residual "wound" from the myotomy is stained blue. After myotomy, the cut ends of the muscle retracts laterally as suggested by the curved lines. Compare with the muscle bulge in Photo 3.
Three months post-op (7 of 8)
Nearly 3 months after myotomy, both the patient and his wife say swallowing is much improved. Note the deep "notch" in the muscle bulge as compared with photo 3.
Three months post-op (7 of 8)
Nearly 3 months after myotomy, both the patient and his wife say swallowing is much improved. Note the deep "notch" in the muscle bulge as compared with photo 3.
At close range (8 of 8)
At very close range with some clockwise rotation of the view. The muscle can no longer impede passage of food or liquid into the esophagus.
At close range (8 of 8)
At very close range with some clockwise rotation of the view. The muscle can no longer impede passage of food or liquid into the esophagus.
Tagged Photos